Remission in aggressive B-cell lymphomas is increasingly linked to complex karyotypes and gene rearrangements that often portend aggressive behavior and resistance to standard therapy. An understanding of lymphoma cytogenetics has lagged behind that of leukemia, in which pretreatment karyotype constitutes an independent prognostic determinant for attainment of complete remission. In 2008, the World Health Organization added the category B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma (BCLu-DLBCL/BL), which incorporates aggressive lymphomas with variable morphology. However, this category is nonspecific and does not elevate cytogenetic criteria to a diagnostic status based on chromosomal abnormalities as seen in acute leukemia. In part, this could be because cytogenetic analyses or fluorescence in situ hybridization (FISH) studies are not routinely performed in diagnosing lymphomas. Double-hit lymphoma (DHL) is traditionally defined as a dual gene rearrangement containing MYC and another gene, usually BCL2. Triple- hit lymphoma (THL) involves an additional rearrangement, which is usually BCL6. DHL and THL always contain complex karyotypes and can be seen in diffuse large B-cell lymphoma (DLBCL) or the newly created BCLu-DLBCL/BL. They are not seen in Burkitt lymphomas, which, by definition, always have simple karyotypes.1 DHL and THL typically present at a median age of 69 years and have a higher prevalence of extranodal and bone marrow involvement as well as central nervous system involvement.2 These characteristic features are responsible for their significantly shortened disease-free survival and overall survival (OS) when compared with other lymphomas in the same category, and thus their detection may have significant therapeutic implication. We propose that consensus use of FISH and/or immunohistochemical (IHC) analysis to detect MYC, BCL2, and BCL6 would lead to improved categorization, better study designs, and, ultimately, improved treatments for patients with aggressive lymphomas.
Case 1: Transformed
A 42-year-old female presented with inguinal adenopathy in October 2010. Excisional biopsy and staging demonstrated grade 2 follicular lymphoma, stage 3A. She received 6 cycles of rituximab, cyclophosphamide, vincristine, and prednisone from November 2010 through March 2011 and achieved a partial remission. Surveillance imaging in September 2011 demonstrated progression of axillary, periportal, retroperitoneal, and inguinal lymphadenopathy. Excisional biopsies in both January 2012 and November 2012 reconfirmed follicular lymphoma grade 2. In December 2012, she began treatment with the PI3 kinase ? inhibitor, idelalisib. Over the following 2 months she noted a slight reduction of supraclavicular and axillary lymphadenopathy. In early January 2013, she developed profound fatigue and petechiae. An initial evaluation was most significant for anemia and thrombocytopenia, with a hemoglobin reduction from 14 g/dL to 7.5 g/dL and a platelet reduction from 140K to 9K. A bone marrow biopsy performed at that time is shown in the Table along with the laboratory results.
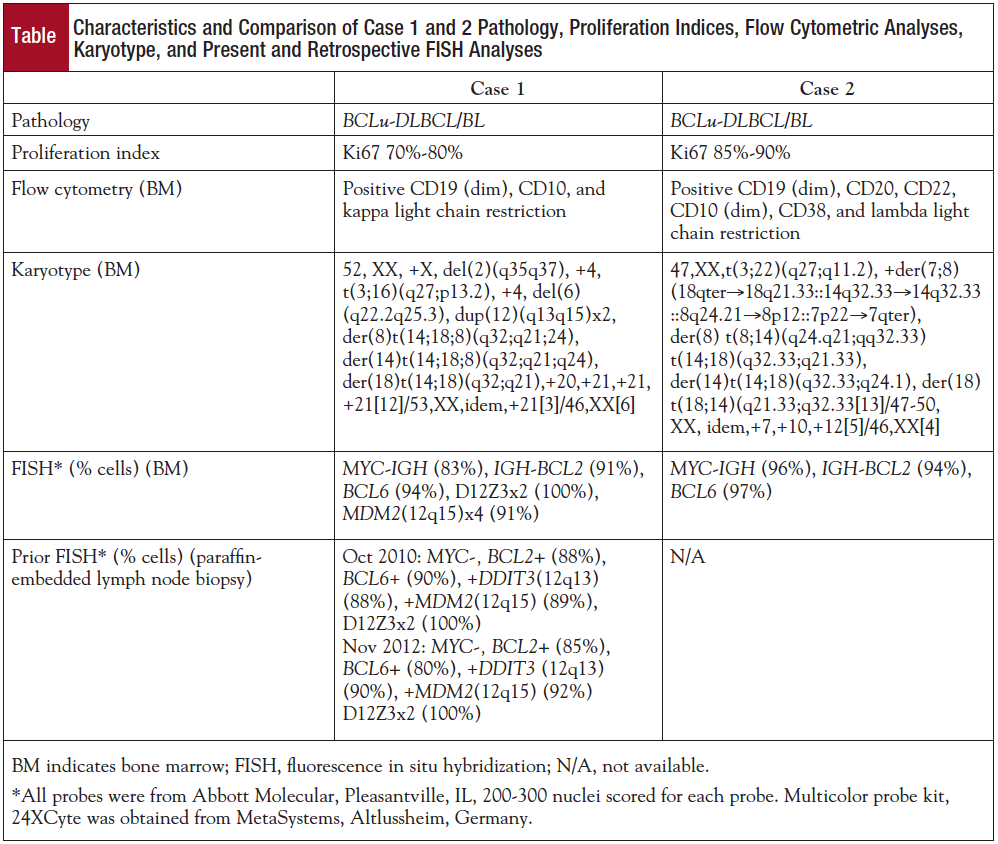
The cytogenetic analysis of Giemsa-banded bone marrow aspirate cells described in the Table is also illustrated in panel A of the Figure. Interphase FISH analysis revealed MYC, BCL2, and BCL6 rearrangements (Table; Figure, panel B). Metaphase FISH analysis confirmed MYC-IGH fusion with colocalization of MYC and IGH on derivative chromosomes 8 and 14, as well as 1 IGH signal on 18q21 (Figure, panel B). Moreover, FISH results also revealed IGH-BCL2 fusion on 2 copies of derivative chromosome 18 and an unexpected colocalization of IGH and BCL2 on the derivative chromosome 8. As shown in panel D in the Figure, there was a remaining IGH signal on the derivative chromosome 14, indicating that IGH locus must have been broken at 2 locations. Additionally, metaphase FISH with BCL6 confirmed that 3? BCL6 remained on 3q27 whereas the 5? BCL6 moved to the 16p31 region as a result of translocation between the long arm of chromosome 3 and the short arm of chromosome 16 (Figure, panel C).
Paraffin-embedded lymph node tissue was obtained from the patient’s prior lymph node biopsies from October 2010 and November 2012 to retroactively evaluate the order of gene rearrangement occurrences. MYC-IGH fusion was not detected in either pretransformation biopsy, but BCL2 and BCL6 rearrangements were detected in both pretransformation samples. Moreover, interphase FISH with 2 probes covering loci on the long arm of chromosome 12 demonstrated 3 copies of DDIT3 (12q15) and MDM2 (12q13), consistent with formation of 12q duplication already present in 2010. However, FISH studies of the bone marrow with these 2 probes confirmed 4 copies of MDM2 and DDIT3 and 2 copies of centromere 12, confirming that the 12q13-q15 chromosomal region was present in 4 copies in the bone marrow cells.
The patient received 1 cycle of rituximab plus cyclophosphamide, doxorubicin, vincristine, methotrexate, leucovorin, filgrastim, intrathecal cytarabine, intrathecal methotrexate, ifosfamide, etoposide, and cytarabine yielding a complete remission, and then successfully underwent allogeneic stem cell transplantation from a matched related donor. She is alive and without evidence of disease 3 months posttransplant and 7 months after diagnosis of THL.
Case 2: De Novo
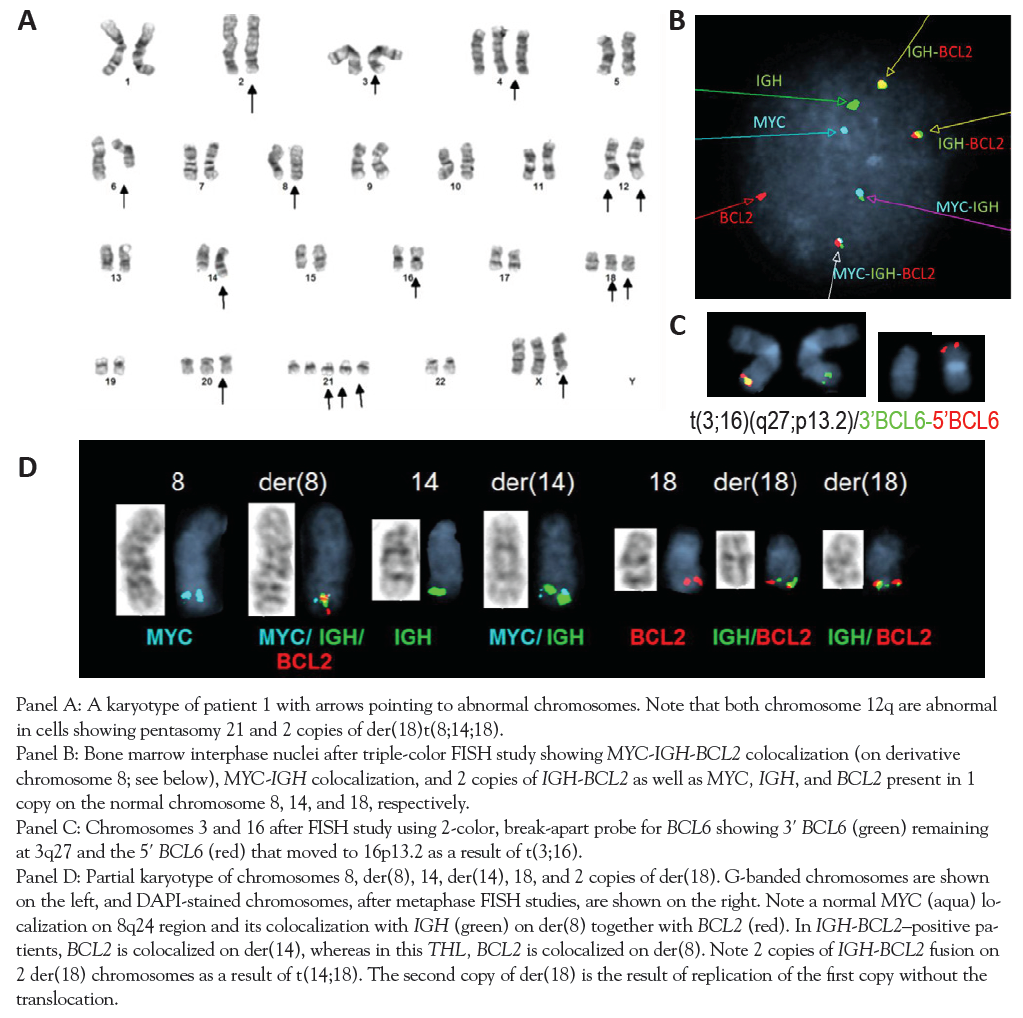
A 58-year-old Trinidadian female developed fatigue, malaise, and abdominal symptoms in April 2013 prior to seeking care in the United States. In June 2013, laboratory testing revealed pancytopenia and renal failure with elevated markers of tumor lysis syndrome. Whole-body CT imaging revealed conglomerate mesenteric lymph nodes, splenomegaly, and encasement of both renal hila, but without hydronephrosis. As shown in panel E of the Figure and in the Table, the karyotype was complex and involved a gain of 8q translocated to the deleted chromosome 7 resulting in trisomy 7q and trisomy 8q, including a third copy of BCL2-IGH fusion. Interphase FISH results were consistent with an atypical MYC-IGH fusion as the result of a third fusion with an additional IGH signal as well as IGH-BCL2 and BCL6 rearrangements. She received rituximab plus cyclophosphamide, doxorubicin, vincristine, dexamethasone, and filgrastim after emergent intubation for altered mental status and respiratory failure but quickly succumbed to multiorgan failure.
Discussion
These 2 cases of rapidly evolving THL were identified at our institution within 6 months of each other. The transformation event for case 1 was caught relatively early and had a favorable outcome. FISH analysis confirmed a suspicion that IGH-MYC was the ultimate
rearrangement and was concomitant with histologic transformation suggesting its causality. MYC-IGH translocation is known to be more aggressive than MYC non-IGH translocations.3 Two breakpoints within the IGH gene indicate that this was a complex rearrangement enhanced by duplication of derivative chromosome 18 and of 12q. Duplication of derivative chromosome 18 as well as of IGH-BCL2 is similar to those seen in duplications of the Philadelphia chromosome in the blast crisis of chronic myelogenous leukemia (CML), which is known to confer a more aggressive phenotype. The mechanism is through the replication of the derivative chromosome 18 and IGH-BCL2, without another translocation. BCL6 rearrangements have not been described in healthy individuals; therefore, BCL2 was presumably the first event. BCL6 has 28 known translocation partners, and t(3;16)(q27;p13) has been described previously.4 Therefore, in this patient, the initial events most likely involved rearrangements of BCL2 and BCL6 as well as duplication of 12q, all detected in the lymph node biopsy in 2010, whereas later events involved MYC and other abnormalities, particularly pentasomy 21 and tetrasomy of the 12q13-q15 region.
Case 2 was not diagnosed early enough to benefit from treatment intervention. The complex karyotype in this patient involved a gain of 8q translocated to chromosome 7 resulting also in 3 copies of IGH-BCL2, reminiscent of duplicated BCR-ABL1 in the blast crisis of CML associated with a more aggressive phenotype. Previous reports illustrate dismal outcomes. Tomita et al published a case series of aggressive lymphomas that included 7 patients with THL whose median OS was 4 months.5 The National Comprehensive Cancer Network 2013 guideline recommends using FISH to identify DHL/THL for specific diagnostic scenarios, although this has not become standard practice.6
Recent publications regarding DHL have examined the methods of detection (FISH vs IHC). Two independent groups have validated the use of IHC to define DHLs (or THLs), and others have validated its use in prognostication.7-9 Whereas IHC identifies 21% of DLBCL as DHL with a 1-year mortality of 25% to 35%, FISH identifies 5% of DLBCL as DHL with a 1-year mortality of 55%.9 Horn et al found that a combined IHC/FISH score was predictive of outcome independent of the International Prognostic Index.10 Their analysis of 442 de novo DLBCL tumor samples for MYC, BCL2, and BCL6 protein expression (IHC) and gene rearrangements (FISH) found that inferior survival was associated with low BCL6 expression, elevated MYC and BCL2 expression, and MYC gene rearrangements. The authors suggested MYC staining as a screening tool followed by FISH testing for all cases with MYC protein IHC expression >20%. This is a reasonable and cost-effective approach to diagnosing multiple-hit lymphomas, although interinstitutional variability in MYC expression positivity is unclear.
Conclusion
Our brief review highlights the crucial role FISH and IHC should play in the evaluation of aggressive lymphomas in both the transformed and de novo setting. Improved outcomes with aggressive lymphomas may be possible with early diagnosis by FISH screening. A role for IHC in identification of aggressive lymphoma phenotypes has also emerged and should be used concomitantly with FISH. Lymphoma pathophysiology has evolved. As such, we know many of the molecular mechanisms that determine our patients’ chance for survival and now have an opportunity to exploit that knowledge for our patients’ benefit.
References
- Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419-2430.
- Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3439-3443.
- Lin P, Dickason T, Fayad LE, et al. Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer. 2012;118:1566-1573.
- Huret JL. BCL6 (B-Cell Lymphoma 6). Atlas Genet Cytogenet Oncol Haematol. 2013;17(6)371-379.
- Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009;94:935-943.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Hodgkin’s Lymphoma. Version 1.2013. www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed July 7, 2013.
- Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460-3467.
- Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273-2279.
- Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452-3459.
- Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253-2263.
Dr McFarland is a Clinical Fellow in the Division of Hematology/Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai in New York, NY. Dr Brody is an Assistant Professor of Medicine in hematology and medical oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai in New York, NY. Joseph Tripodi is an NYSDOE- and ASCP-certified research staff member at the Tumor CytoGenomics Laboratory at the Icahn School of Medicine at Mount Sinai in New York, NY. Issa Leonard is an NYSDOE- and ASCP-certified Lab Coordinator at the Tumor CytoGenomics Laboratory at the Icahn School of Medicine at Mount Sinai in New York, NY. Dr Najfeld has been Director of the Tumor CytoGenomics Laboratory since 1980 and is internationally known in the field of cancer cytogenetics, specifically for chronic myeloproliferative disorders.

