In the United States, colorectal cancer (CRC) is the fourth most common malignancy, with 134,490 new cases and 49,190 deaths estimated in 2016.1 Among these patients, 25% present with advanced disease at diagnosis, and nearly 50% will eventually develop metastatic disease.2 Survival rates for metastatic CRC (mCRC) have been improving, with the most recent clinical trials reporting overall survival (OS) rates of almost 30 months.3 Over the past decade, there has been an increased focus on personalized and more patient-centered oncologic care. This personalization is occurring in the therapeutic approach and the overall delivery of care to patients with cancer.
Anticancer therapy is evolving from nonspecific cytotoxic drugs, which damage malignant as well as normal cells, to more specific targeted agents and immunotherapy. Targeted and immune-based therapies using biomarkers strive for more precise efficacy with less toxicity by assigning specific treatments to subgroups of patients who are most likely to benefit. With the advent of next-generation sequencing technology, the newfound ability to efficiently sequence individual cancer genomes opens a new chapter in cancer research, with the potential for expanding tailored oncologic care.
Despite overall improvements in therapy, however, our understanding of why some patients with CRC respond to therapy and others do not remains poor. The clearest evidence of the benefit from personalized therapeutics is the use of mismatch repair (MMR) gene status for treatment decisions, as well as the use of epidermal growth factor receptor (EGFR) inhibitors in the RAS wild-type (WT) population. This article will focus on the current and future treatment paradigms of CRC in relation to personalized medicine, including opportunities for personalization in therapeutics as well as the delivery of treatment and care.
Molecular Biology of CRC
CRC is a heterogeneous and complex malignancy that involves many signaling pathways.4 Histologically, identical tumors often respond differently to therapy and prognosis. Thus, significant tumor heterogeneity, not only within the primary tumor but also at metastatic sites, presents a challenge when developing personalized medicine.5
Location of tumor (right vs left) has been shown to impact outcomes.6,7 In a retrospective analysis of the phase 3 CALGB/SWOG 80405 trial, 1137 patients with KRAS WT mCRC were analyzed based on their location of right (cecum to hepatic flexure) versus left (splenic flexure to rectum) side.6 In the overall study of CALGB/SWOG 80405, no difference in OS or progression-free survival (PFS) was found when bevacizumab or cetuximab was added to first-line treatment with 5-fluorouracil plus oxaliplatin (FOLFOX) or irinotecan/5-fluorouracil/leucovorin (FOLFIRI) in patients with mCRC.8 However, patients with tumors originating in the right side of the colon had shorter median OS (19.4 months) than patients with left-sided tumors (33.3 months; hazard ratio [HR] = 1.60; P <.001). This study also showed the relative efficacy of cetuximab and bevacizumab depending on tumor location. Among patients who received cetuximab, median OS was 36 months for patients with left-sided tumors but only 16.7 months for those with right-sided tumors. With bevacizumab, median OS was 31.4 months and 24.2 months in left versus right, respectively.6 These data and other similar reports evaluating tumor location have led the National Comprehensive Cancer Network (NCCN) to recommend the use of cetuximab and panitumumab as initial therapy only for KRAS/NRAS WT patients whose primary tumors originated on the left side of the colon.9 Currently, this recommendation does not apply to second-line therapy and beyond.
There are many proposed mechanisms by which CRC is thought to occur. The chromosomal instability pathway involves “gain of function” mutations. These tumors may be either inherited (as with familial adenomatous polyposis) or sporadic. The initial step in tumorigenesis is adenoma formation, associated with loss of the adenomatous polyposis coli gene. As adenomas become larger and evolve to early carcinoma, they acquire mutations in the small GTPase KRAS, followed by loss of chromosome 18q with SMAD4, which is downstream of transforming growth factor beta (TGF-β), and mutations in TP53 in carcinoma. These changes result in chromosomal instability (CIN). CIN, which is defined as the presence of multiple structural or numerical chromosome changes in a tumor cell, is present in approximately 65% to 70% of cases of CRC. In a meta- analysis, the overall HR for death associated with CIN was 1.45 (95% confidence interval [CI], 1.35-1.55; P <.001). The effect was similar for PFS (HR = 1.71; 95% CI, 1.51-1.94; P <.001).10
Tumors that have microsatellite instability (MSI), however, carry these changes infrequently; therefore, the development of CRC involves alternative genetic changes that also lead to CIN. The MMR pathway is the pathway involved in hereditary nonpolyposis CRC, also called Lynch syndrome, which occurs in only 2% to 3% of CRC.11 These patients have a germline loss of the DNA MMR genes that results in accumulation of DNA defects, most commonly in microsatellite areas. This leads to an MSI-high (MSI-H) phenotype.12 Approximately 15% of patients with CRC will have an MSI-H phenotype, but unlike in Lynch syndrome, this occurs through hypermethylation of the promoter region of some MMR genes and/or DNA hypomethylation with loss of imprinting (ie, silencing of gene expression).13,14
The third proposed pathway is the hypermethylation phenotype (CpG island methylator phenotype [CIMP+]) pathway. These tumors involve epigenetic alterations such as hyper- or hypomethylation and loss of imprinting, which can silence the expression of necessary genes, such as MMR enzymes.15
The Cancer Genome Atlas (TCGA) Research Network published a comprehensive molecular characterization of CRC in 2012.16 The report found that almost 100% of commonly affected signaling pathways or genes had changes in MYC transcriptional targets; 93% of tumors had an alteration in the WNT pathway; 55% of nonhypermutated tumors had alterations in KRAS, BRAF, or NRAS; and 33% had alterations in both the phosphoinositide 3-kinase and RAS pathways. Also reported in this analysis was deletion of other important tumor suppressor genes (ie, phosphatase and tensin homolog and gene amplifications (including IGF2 and ERBB2). To resolve inconsistencies in classification of gene expressions in CRC and understand variation in treatment responsiveness, an international consortium was formed to develop a more robust classification system (Figure).17
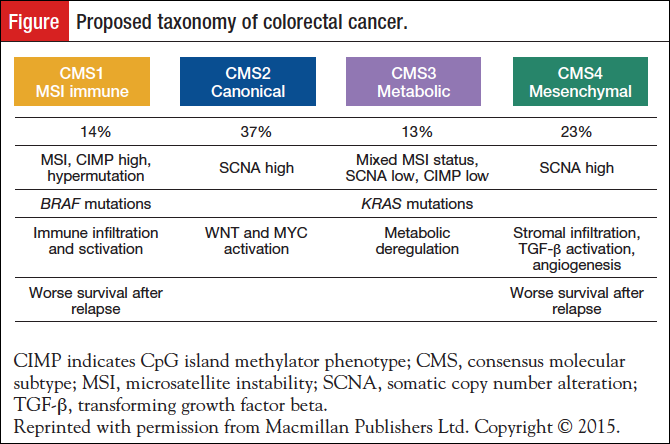
Utilizing multiple data sets, including TCGA, they described 4 consensus molecular subtypes (CMSs) with distinguishing features: (1) CMS1 (MSI immune, 14%), hypermutated, microsatellite unstable and strong immune activation; (2) CMS2 (canonical, 37%), epithelial, chromosomally unstable, marked WNT and MYC signaling activation; (3) CMS3 (metabolic, 13%), epithelial and evident metabolic dysregulation; and (4) CMS4 (mesenchymal, 23%), prominent TGF-β activation, stromal invasion, and angiogenesis. CMS1 tumors were frequently diagnosed in women with right-sided lesions and had a higher histopathologic grade at diagnosis. Conversely, CMS2 tumors were left-sided, and CMS4 tumors were diagnosed at a higher stage (either III or IV). In multivariate analysis, after adjustment for clinicopathologic features (ie, MSI status, and KRAS, BRAF mutations), CMS4 tumors resulted in worst OS and relapse-free survival. In addition, superior survival rates were noted in CMS2 patients.
Current Treatment Paradigm for mCRC: Available Agents
Many treatment combinations are available for patients with metastatic disease, and standard cytotoxic treatment includes a fluoropyrimidine-backbone (intravenous 5-fluorouracil or oral capecitabine) in combination with oxaliplatin or irinotecan.18,19 TAS-102, an orally administered combination of a thymidine-based nucleic acid analog (trifluridine) and a thymidine phosphorylase inhibitor (tipiracil hydrochloride), is a recently approved cytotoxic option in the refractory setting.20 There are currently no prospectively validated predictive biomarkers for conventional cytotoxic chemotherapy agents.
An increasing number of targeted therapies have become available for use in combination with standard cytotoxic therapies that are not biomarker-driven. Inhibition of vascular endothelial growth factor (VEGF) has proven to be an effective approach in mCRC. Bevacizumab, a monoclonal antibody targeting VEGF, has demonstrated efficacy in combination with FOLFOX21 and FOLFIRI18 in patients with mCRC. Aflibercept is a recombinant fusion protein consisting of VEGF binding portions from key domains of human VEGF receptors (VEGFRs) 1 and 2 fused to the Fc portion of human immunoglobulin (Ig) G1. It functions as a decoy receptor that prevents intravascular and extravascular VEGF-A, VEGF-B, and placental growth factor from binding to their receptors. Aflibercept is approved for use in combination with FOLFIRI in patients who have progressed on an oxaliplatin-containing regimen.22 Ramucirumab, a recombinant monoclonal antibody of the IgG1 class that binds to VEGFR-2, blocking receptor activation, is approved for second-line treatment of mCRC after progression on bevacizumab, oxaliplatin, and fluoropyrimidine.23 Regorafenib (BAY 73-4506) is an orally active multikinase inhibitor that inhibits the activity of several protein kinases implicated in the regulation of tumor angiogenesis (VEGFR-1 [FLT-1], VEGFR-2 [KDR], VEGFR-3 [FLT-4], TIE-2 [TLK]), oncogenesis (KIT, RET, BRAF, and BRAFV600E), and the tumor microenvironment (platelet-derived growth factor receptor and fibroblast growth factor receptor) and is utilized in the refractory setting.24
The discovery that abnormalities in EGFR contribute to the development and growth of CRC was a pivotal point for true personalization of treatment based on the molecular characteristics of an individual colon tumor. The EGFR inhibitors cetuximab25 and panitumumab26 are the first FDA-approved agents for the treatment of CRC that utilize a specific personalized biomarker, RAS.
Therapeutic Targeting in CRC: EGFR Inhibitors and Other Agents Under Investigation
The RAS oncogene has 3 variants, HRAS, KRAS, and NRAS, with KRAS being the most commonly mutated in CRC.27-29 The RAS oncogenes encode a group of small proteins with homology to G-proteins that regulate cellular signal transduction. RAS mutation, most commonly a point mutation, leaves the protein resistant to hydrolysis, which results in a constitutively active protein and continuous growth stimulus.30 It is now well established that activating mutations in KRAS result in resistance to the anti-EGFR therapies cetuximab and panitumumab. Activating mutations are found in more than 40% of cases of mCRC, with concordance between the primary and synchronous distant metastasis31,32; however, the rate of discordance between primary and recurrent tumors may be up to 20%.33
Cetuximab, a mouse/human chimeric monoclonal antibody, binds to EGFR in both tumor cells and normal cells, competitively inhibiting ligand binding and inducing receptor dimerization and internalization.34 It has been approved in the first-line,35-37 second-line,38 and refractory settings,25,39 in combination with irinotecan25,35,38 and as monotherapy.39,40 The combination of cetuximab plus oxaliplatin has had mixed results, and therefore the benefit of adding cetuximab to a first-line oxaliplatin-based regimen is less certain.37,41,42 Panitumumab is a fully human monoclonal antibody with efficacy similar to that of cetuximab when used as a single agent in refractory metastatic disease, and when used in first- and second-line settings in combination with irinotecan-containing chemotherapy.43 In addition, panitumumab has been shown to result in fewer infusion reactions than have been observed with cetuximab.
The initial FDA approval for panitumumab and cetuximab was for use in tumors without KRAS mutations in exon 2 (codon 12, 13).44-47 Response rates, however, are 40% or less in unselected populations, and it has become clear that mutations in KRAS outside of exon 2 and NRAS also identify a population that will not benefit from an anti-EGFR agent.48-50 In the Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) trial, 1183 patients with previously untreated mCRC were randomly assigned to FOLFOX4 with or without panitumumab. In the study population, 108 (17%) patients without exon 2 KRAS mutations had other mutations in RAS.50 These additional mutations predicted a lack of response to panitumumab, and their presence was actually associated with inferior PFS and OS in patients receiving panitumumab plus FOLFOX4 compared with FOLFOX4 alone, although the difference was not significant.
CALGB/SWOG 80405 initially recruited KRAS-unselected patients with mCRC who received treatment according to physician-selected chemotherapy (FOLFIRI or modified FOLFOX6) and were randomized to cetuximab, bevacizumab, or both (the third arm was subsequently closed). After 1420 patients were accrued, the study was amended to select only patients with WT KRAS tumors (codons 12 and 13). Expanded RAS was tested retrospectively in all WT KRAS exon 2 patients and included KRAS exons 3, 4 and NRAS exons 2, 3, and 4. In the expanded RAS WT population, the median OS extended beyond 30 months. However, there was no significant difference between the chemo+cetuximab arm and the chemo+bevacizumab arm (32 months vs 31.2 months), nor was there a difference in PFS. However, there was a higher overall response rate (ORR) achieved in the cetuximab arm in the expanded RAS population (68.6% vs 53.6%; P <.01).51
The phase 3 FIRE-3 trial was conducted at 150 German and Austrian cancer centers and compared FOLFIRI plus either cetuximab or bevacizumab as first-line treatment in patients with mCRC who had KRAS WT disease.3 The 2 groups had similar ORR and PFS, but OS significantly favored the cetuximab-FOLFIRI combination over bevacizumab-FOLFIRI. As part of a preplanned analysis, tumors were evaluated for extended RAS testing. Among the 592 patients in the trial, 407 had tumors that could be evaluated for all of the additional mutations of interest (KRAS exons 3, 4 and NRAS exons 2, 3, 4). The rate of extended RAS mutations in this group was 16%, with KRAS exon 4 being the most common. The addition of cetuximab to FOLFIRI showed an increased median survival compared with bevacizumab-FOLFIRI: 33.1 months (95% CI, 24.5-39.4) versus 25.0 months (95% CI, 23.0-28.1); HR = 0.70 (range, 0.54-0.90; P = .0059) in the extended RAS WT population.52
The further identification of these predictive biomarkers has led the American Society of Clinical Oncology (ASCO)53 and the NCCN9 to recommend that all patients who are candidates for anti-EGFR therapy have their tumor tested for mutations in both KRAS and NRAS exons 2 (codons 12 and 13), 3 (codons 59 and 61), and 4 (codons 117 and 146). In addition, evidence increasingly suggests that response to EGFR-targeted agents is unlikely in patients whose tumors harbor BRAF mutations (particularly the BRAFV600E mutation). Two meta-analyses have addressed the efficacy of EGFR antibody therapies in patients with RAS WT/BRAF-mutated tumors, and neither analysis found a survival advantage for the addition of EGFR antibody therapy in this population.54,55
The human EGFR2 (HER2) oncogene encodes for a transmembrane glycoprotein receptor that functions as an intracellular tyrosine kinase. In breast56 and gastroesophageal cancers,57 targeted therapies who block HER2 (ie, trastuzumab, lapatinib, pertuzumab) have become important options for patients who overexpress HER2 in their tumor. In CRC, amplification of HER2 is rare.58 A phase 2 study evaluated the efficacy of dual-targeted therapy with trastuzumab plus lapatinib in patients with KRAS WT, HER2-overexpressing mCRC. At a median follow-up of 94 weeks, there were 8 (30%) objective responders, 1 complete responder, and 12 (44%) patients with stable disease.59 These data may provide proof of principle, but further studies are needed to confirm this approach.
Prognostication in CRC: BRAF and MMR Genes
There are clinical, histologic, and molecular features that may influence prognosis of CRC, independent of stage. Understanding these prognostic factors allows oncologists to give patients a more precise estimate of prognosis based on their specific characteristics. Factors such as presence of distant metastasis, local tumor extent, nodal positivity, lymphovascular and perineural invasion, and clinical obstruction or perforation contribute to prognosis. Further stratification can be achieved by identification of those with a high preoperative serum level of the tumor marker carcinoembryonic antigen, with levels ≥5.0 ng/mL having an adverse impact on survival.60 BRAF-activating mutations, specifically BRAFV600E, occur in less than 10% of sporadic CRCs and are a strong negative prognostic marker for early-stage and advanced tumors with intact MMR function.61-63 In tumors with deficient MMR activity, in which most of the BRAF mutations occur, the presence of a mutation does not have the same adverse prognostic significance.64 Despite efficacy in melanoma, inhibition of BRAFV600E has not proven beneficial in CRC. Ongoing studies are assessing combinations of BRAF inhibitors with MEK, EGFR, or PI3K inhibitors65,66 to overcome resistance mechanisms based on initial promising data from early-phase clinical trials.
Identification of either germline mutations or epigenetic changes in MMR genes has enabled more personalized identification of hereditary forms of CRC and may have therapeutic implications. MMR genes are responsible for correcting nucleotide base mispairings, deletions, or insertions that can occur during DNA replication.13 Germline mutations in one of the MMR genes is the underlying genetic defect in Lynch syndrome, and loss of expression of MMR genes can also be found in approximately 15% of sporadic CRC. Identification of Lynch syndrome is critical to counsel patients on surgical options and expanded surveillance for future malignancies. In addition to the issue of familial cancer risk, deficient MMR is a prognostic biomarker associated with a lower recurrence risk in patients with stage II CRC and also a predictive biomarker for lack of benefit, and potentially even harm, from adjuvant single-agent fluoropyrimidine chemotherapy.67,68
As recently reported, mutations or epigenetic changes in MMR genes may also have implications for efficacy of immunotherapy, allowing for an additional personalized biomarker in the treatment of advanced disease. Tumors that lack MMR mechanisms have many more mutations (hypermutated) when compared with tumors with intact MMR function.69,70 Tumors with more mutations generate neoantigens that are capable of being recognized as “non-self” immunogenic antigens. To effectively attack tumor cells, the immune system must recognize the tumor, present the antigen to T cells, activate T cells, and directly attack the tumor. There are several checkpoints in the immune system to dampen this response to protect against autoimmunity; however, in the setting of malignancy, these immune checkpoints can lead to tolerance of the tumor and thus to progression.
One of the checkpoints that has been the focus of therapeutic targeting is the programmed death 1 (PD-1) receptor and its ligand (PD-L1). In a phase 2 study, 41 patients with progressive metastatic carcinoma with or without MMR deficiency were treated with pembrolizumab, an anti–PD-1 inhibitor. The ORR was 40% in patients with deficient MMR and 0% in those with intact MMR.70 Benefit from immunotherapy in patients with MMR-deficient tumors was also suggested in the CheckMate-142 trial, in which patients with MMR-deficient (n = 59) or MMR-proficient (n = 23) mCRC received nivolumab (a fully human anti–PD-L1 monoclonal antibody) with or without ipilimumab, a monoclonal antibody directed against cytotoxic T-lymphocyte antigen 4. In this study, preliminary reports indicate that immunotherapy benefited those with MMR-deficient tumors (13 confirmed partial responses; median PFS, 5.3 months), and there were no objective responses among those with MMR-proficient tumors (median PFS, 1.4 months).71 The use of immunotherapy in MMR-deficient tumors will need additional investigation in randomized studies, but these data show promise for further personalization of therapy based on MMR status in CRC.
Despite the predictive and/or prognostic value found with RAS mutations, BRAF, MMR genes, and potentially HER2, there are many other markers that have not shown conclusive evidence of benefit: p53,72 loss of heterozygosity at chromosome 18,73 loss of 18q,74 and markers of proliferation (ie, evaluation of S phase).73
Molecular Testing As a Means for Personalization
Increasingly, biomarker expression is driving decision-making to offer patients a more personalized therapeutic approach and potentially improved clinical outcomes. In the adjuvant setting, gene-expression profiling—using either Oncotype DX® (Genomic Health) or the ColoPrint® assay (Agendia)—attempts to identify molecular signatures that provide an accurate and personalized assessment of risk of relapse and benefit of chemotherapy. These assays offer information on prognosis but thus far have not provided predictive data as to the benefit of adjuvant therapy, which limits their use.75
The development of next-generation sequencing techniques has allowed a more affordable and faster method of performing genetic sequencing in hopes of finding targetable alterations.76 One of the challenges of molecular profiling is the oftentimes low frequency of specific molecular changes in a particular malignancy type. Well-designed clinical trials that collect tissue samples are critical to more robustly study these selected groups of patients. The largest trial utilizing targeted therapy, NCI-Molecular Analysis for Therapy Choice (NCI-MATCH), activated in August 2015, is currently ongoing and includes approximately 2400 sites across the United States. This clinical trial sequences tumor biopsies to identify genetic abnormalities that may respond to the targeted drugs selected for the trial. The primary end point for NCI-MATCH is ORR, with secondary end points of PFS, time to progression, and side effects. This trial is quickly accruing patients and has the potential to make significant progress in advancing precision medicine for patients with multiple tumor types, including CRCs.
Personalizing Care Delivery for Patients with CRC
As we strive to offer more personalized therapeutic options for patients with CRC, we also aim for personalized and patient-centered healthcare delivery.
Contemporary CRC care is complex and requires application of multiple diagnostic and treatment modalities, involving numerous specialties along the care continuum.77 An individual patient’s optimal care depends on multiple factors, such as disease stage, prognosis, clinical characteristics, tumor molecular profile, and patient preferences, and may require a combination of modalities, including imaging, pathology testing, molecular profiling, surgery, genetic counseling, hereditary syndrome testing, fertility preservation, oncotherapy, clinical trial participation, comorbidity care, psychosocial support, palliative care, and others.9,78 Personalization of the planning and delivery of CRC care is essential to the timely and effective provision of all indicated care services79; however, it is challenged by fragmentation and the lack of coordination across clinical domains and provider organizations—difficulties contributing to the situation that the Institute of Medicine (IOM) described as “a system in crisis.”80
New care delivery models have emerged in recent years in response to calls from the IOM, ASCO, and others to address fragmentation and provide patient-centric, coordinated cancer care. The models include patient navigation, financial counseling, oncology medical home, team-based care, and various approaches to patient engagement.81 Although each of these models represents an important step toward coordinated patient-centric cancer care, they address a key requirement of personalized cancer care planning and delivery, which is timely management of interdependent care across clinical domains.82 To ensure delivery of the “right care to the right patient at the right time,” interdependent care events must be planned and delivered at a specific time and in a specific order/sequence. CRC care provides numerous examples of interdependent care that is challenging to orchestrate in the “right” sequence and at the “right” time. They include RAS mutation testing in time for decision on the first line of therapy for metastatic disease; genetic counseling and Lynch syndrome testing in time to inform a surgical strategy for operable CRC; fertility preservation treatment completed prior to the start of an oncotherapy that compromises patient reproductive functions; early psychosocial screening and care to avoid distress escalation; and enrollment in clinical trials prior to the first line of therapy, for trials accruing treatment-naive patients.
Orchestrating the relative timing and sequencing of these and similar interdependent care events in CRC, as well as other cancers with complex care delivery, requires a systematic methodology and approach, beyond patient navigation and provider communication.79,82-84 Following IOM recommendation to apply a systems engineering methodology to solve care coordination problems,85 it has been proposed to use the project management discipline to plan and deliver care for diseases such as CRC that require management of interdependent care.82,86-89 Project management is widely used in many industries for systematic timing and sequencing of complex interdependent events and coordinating activities across dynamic multidisciplinary teams.90-93 In the cancer setting, this methodology can be used to plan and manage each patient’s care as a project, personalized to the patient’s clinical indications, disease characteristics, and preferences. This approach is termed the 4R Oncology Model—Right Information and Right Care to the Right Patient at the Right Time88—and has been highlighted as part of the NCI-ASCO Teams in Cancer Care Delivery initiative.82 This emerging approach to facilitating personalized care in cancer planning and delivery has been described in the context of breast cancer, and pilots in breast cancer are currently underway at 3 cancer centers.94 Patients with CRC could derive considerable benefit from the application of the 4R approach, and implementation of 4R in CRC should be a priority.
As the number of cancer survivors increases, there has been growing focus on survivorship care as a method to provide coordinated and patient-centered care after completion of cancer therapy. Cancer survivors face lifelong surveillance for recurrence and secondary cancers, in addition to experiencing a host of physical and psychosocial late and long-term effects that can extend years after completing treatment and which must be managed along with comorbid conditions often deprioritized during cancer treatment.95,96 Due to fragmentation within the healthcare system, which results in inadequate care coordination across providers, many patients have difficulty finding appropriate resources and information to address their concerns.97 IOM, ASCO, and other leading organizations have recommended the provision of survivorship care plans at the end of cancer treatment to improve coordination of ongoing clinical care. These plans are written documents comprising comprehensive treatment summaries, recommendations for detailed surveillance and follow-up care, links to support services, and health promotion information that is shared with the patient and primary care provider.
Conclusion
To truly personalize and provide comprehensive care for patients with CRC, all stakeholders must collaborate, including patients, providers, health systems, pharmaceutical companies, insurance plans, and government agencies. In an era of rapidly growing knowledge of the molecular basis of cancers, integration of this expanding knowledge with effective models of healthcare delivery will be critical to improving outcomes of patients with colorectal cancer.
Source of Funding
This research was supported by grants from the National Cancer Institute: U10CA017145 (Dr Kircher, Dr Nimeiri, Dr Benson) and UG1CA189828 (Dr Trosman, Ms Weldon).
Dr Kircher’s work focuses on expanding cancer survivorship services and studying the late effects of chemotherapy.
Dr Nimeiri is an Associate Professor of Medicine at Robert H. Lurie Comprehensive Cancer Center. Her work focuses on clinical trials and biomarker stratification in colorectal cancer.
Dr Trosman is a cofounder and codirector of the Center for Business Models in Healthcare. She conducts health services research of personalized cancer care delivery models and precision oncology adoption and coverage/reimbursement.
Ms Weldon is a cofounder and codirector of the Center for Business Models in Healthcare. Her work is focused on delivery models, tools, and collaborative structure for supportive oncology and precision medicine.
Dr Benson is Professor of Medicine and Associate Director for Cooperative Groups at Robert H. Lurie Comprehensive Cancer Center. He has had national leadership roles in numerous organizations, including the Association of Community Cancer Centers, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network.
References
- SEER Program. Cancer stat facts: colon and rectum cancer. National Cancer Institute website. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed October 17, 2016.
- Van Cutsem E, Nordlinger B, Cervantes A; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21(suppl 5):v93-v97.
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075.
- Deschoolmeester V, Baay M, Specenier P, et al. A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist. 2010;15:699-731.
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460.
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(suppl):Abstract 3504.
- Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results—Medicare data. J Clin Oncol. 2011;29:4401-4409.
- Venook AP, Niedzwiecki D, Lenz HJ. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(suppl 5s):Abstract LBA3.
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Colon Cancer. Version 1.2017. www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed January 3, 2017.
- Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut. 2008;57:941-950.
- Kerber RA, Neklason DW, Samowitz WS, et al. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (Lynch syndrome) in a large population database. Fam Cancer. 2005;4:239-244.
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079-1099.
- Chung DC, Rustgi AK. DNA mismatch repair and cancer. Gastroenterology. 1995;109:1685-1699.
- Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793.
- Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632-4642.
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337.
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer: Irinotecan Study Group. N Engl J Med. 2000;343:905-914.
- Mayer RJ, Van Cutsem E, Falcone A, et al; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
- Emmanouilides C, Sfakiotaki G, Androulakis N, et al. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer. 2007;7:91.
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506.
- Tabernero J, Yoshino T, Cohn AL, et al; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508.
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345.
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713.
- Takayama T, Ohi M, Hayashi T, et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599-611.
- Shibata D, Schaeffer J, Li ZH, et al. Genetic heterogeneity of the c-K-ras locus in colorectal adenomas but not in adenocarcinomas. J Natl Cancer Inst. 1993;85:1058-1063.
- Tortola S, Marcuello E, Gonzalez I, et al. p53 and K-ras gene mutations correlate with tumor aggressiveness but are not of routine prognostic value in colorectal cancer. J Clin Oncol. 1999;17:1375-1381.
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117-127.
- Han CB, Li F, Ma JT, et al. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: a meta-analysis and systematic review. Cancer Invest. 2012;30:741-747.
- Peeters M, Kafatos G, Taylor A, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer. 2015;51:1704-1713.
- Lee KH, Kim JS, Lee CS, et al. KRAS discordance between primary and recurrent tumors after radical resection of colorectal cancers. J Surg Oncol. 2015;111:1059-1064.
- Bardelli A, Janne PA. The road to resistance: EGFR mutation and cetuximab. Nat Med. 2012;18:199-200.
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417.
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019.
- Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546.
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-2319.
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-1765.
- Maughan TS, Adams RA, Smith CG, et al; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114.
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762.
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569-579.
- Jimeno A, Messersmith WA, Hirsch FR, et al. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27:1130-1136.
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634.
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-5712.
- Garm Spindler KL, Pallisgaard N, Rasmussen AA, et al. The importance of KRAS mutations and EGF61A>G polymorphism to the effect of cetuximab and irinotecan in metastatic colorectal cancer. Ann Oncol. 2009;20:879-884.
- Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13-21.
- Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res. 2015;21:5469-5479.
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034.
- Lenz H, Niedzwiecki D, Innocenti F, et al: CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FY/leucovorin (mFOLFOX6) with bevacizumab or cetuximab for patients with expanded ras analyses untreated metastatic adenocarcinoma of the colon or rectum. Presented at ESMO Congress; September 29, 2014; Madrid, Spain. Abstract 5010.
- Stintzing S, Modest DP, Rossius L, et al; FIRE-3 Investigators. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-1434.
- Allegra CJ, Rumble RB, Schilsky RL. Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology provisional clinical opinion update 2015 summary. J Oncol Pract. 2016;12:180-181.
- Rowland A, Dias MM, Wiese MD, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112:1888-1894.
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-594.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792.
- Bang YJ, Van Cutsem E, Feyereislova A, et al; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697.
- Kavanagh DO, Chambers G, O’Grady L, et al. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1.
- Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746.
- Thirunavukarasu P, Sukumar S, Sathaiah M, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689-697.
- Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664-3672.
- Gonsalves WI, Mahoney MR, Sargent DJ, et al; Alliance for Clinical Trials in Oncology. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106:dju106.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-474.
- Seppälä TT, Böhm JP, Friman M, et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer. 2015;112:1966-1975.
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100-103.
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227-235.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247-257.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609-618.
- Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22:813-820.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
- Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol. 2016;34(suppl):Abstract 3501.
- Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92:434-444.
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327.
- Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060-2070.
- Sveen A, Nesbakken A, Ågesen TH, et al. Anticipating the clinical use of prognostic gene expression-based tests for colon cancer stage II and III: is Godot finally arriving? Clin Cancer Res. 2013;19:6669-6677.
- Liu L, Li Y, Li S, et al. Comparison of next-generation sequencing systems. J Biomed Biotechnol. 2012;2012:251364.
- Benson AB III, Chakravarthy AB, Hamilton SR, et al, eds. Cancers of the Colon and Rectum: A Multidisciplinary Approach to Diagnosis and Management Series. 1st ed. New York, NY: Demos Medical Publishing; 2014.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Rectal Cancer. Version 2.2017. www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed June 20, 2016.
- Balogh EP, Ganz PA, Murphy SB, et al. Patient-centered cancer treatment planning: improving the quality of oncology care: summary of an Institute of Medicine workshop. Oncologist. 2011;16:1800-1805.
- Levit LA, Balogh EP, Nass SJ, et al, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013.
- Okun S, Schoenbaum SC, Andrews D, et al. Patients and Health Care Teams Forging Effective Partnerships. Washington, DC: National Academies Press; 2014.
- 82. Trosman JR, Carlos RC, Simon MA, et al. Care for a patient with cancer as a project: management of complex task interdependence in cancer care delivery. J Oncol Pract. 2016;12:1101-1113.
- Trosman J, Weldon C. Models of care delivery. In: Benson AB III, Chakravarthy AB, Hamilton SR, et al, eds. Cancers of the Colon and Rectum: A Multidisciplinary Approach to Diagnosis and Management Series. 1st ed. New York, NY: Demos Medical Publishing; 2014:273-280.
- Weldon CB, Trosman J, Schink JC. Cost of cancer: there is more to it than containing chemotherapy costs. Oncology (Williston Park). 2012;26:1116,1118.
- Reid PP, Compton D, Grossman JH, et al, eds. Building a Better Delivery System: A New Engineering/Health Care Partnership. Washington, DC: National Academies Press; 2005.
- Quinn T. Bringing a management model to healthcare: team-based care. Forbes website. www.forbes.com/sites/groupthink/2014/12/09/bringing-a-management-model-to-healthcare-team-based-care/#601078a533a1. Published December 9, 2014. Accessed February 27, 2017.
- Scher DL. 5 lessons healthcare can learn from project management. Medical Practice Insider website. www.medicalpracticeinsider.com/blog/business/5-lessons-healthcare-can-learn-project-management. Published June 3, 2013. Accessed February 27, 2017.
- Trosman JR, Weldon CB. Company profile: center for business models in healthcare. Per Med. 2013;10:333-337.
- Weldon CB, Friedewald SM, Kulkarni SA, et al. Radiology as the point of cancer patient and care team engagement: applying the 4R model at a patient’s breast cancer care initiation. J Am Coll Radiol. 2016;13(12 pt B):1579-1589.
- Nokes S, Kelly S. The Definitive Guide to Project Management: The Fast Track to Getting the Job Done on Time and on Budget. 2nd ed. Harlow, UK: Pearson Education; 2007.
- Dinsmore PC, Cooke-Davies TJ. Right Projects Done Right: From Business Strategy to Successful Project Implementation. New York, NY: Wiley; 2005.
- Project Management Institute. A Guide to the Project Management Body of Knowledge (PMBOK® Guide). 5th ed. Philadelphia, PA: Project Management Institute; 2013.
- Harvard Business Review. HBR Guide to Project Management. Boston, MA: Harvard Business Review; 2013.
- Lederman L, Madden D, Battle D, et al. Patient advocates collaborate to ensure patients are members of their own oncology care teams. J Oncol Pract. 2016;12:980-982.
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
- Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322-1330.
- Committee on Cancer Survivorship: Improving Care and Quality of Life, National Cancer Policy Board; Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006.

