 Advances in molecular pathology have allowed for the widespread use of sequencing technologies to improve our ability to better understand the biology of each individual patient’s cancer. This allows for the personalization of treatment strategies depending on the molecular profile of the cancer. In colorectal cancer (CRC), mutations in KRAS and NRAS, which cause constitutively active signaling downstream of the epidermal growth factor receptor (EGFR; Figure), have already shown clinical importance for the prediction of resistance to the anti-EGFR therapies cetuximab and panitumumab.1
Advances in molecular pathology have allowed for the widespread use of sequencing technologies to improve our ability to better understand the biology of each individual patient’s cancer. This allows for the personalization of treatment strategies depending on the molecular profile of the cancer. In colorectal cancer (CRC), mutations in KRAS and NRAS, which cause constitutively active signaling downstream of the epidermal growth factor receptor (EGFR; Figure), have already shown clinical importance for the prediction of resistance to the anti-EGFR therapies cetuximab and panitumumab.1
The RAF/MEK/ERK signaling cascade, downstream of EGFR, can also be activated in the presence of BRAF mutations. This mutation is of great clinical interest across many cancer types following the benefit seen for BRAF- or MEK-targeted therapies for the treatment of BRAF-mutant melanoma.2 The Val600Glu (V600E) mutation accounts for 98% of all BRAF mutations and is present in approximately 10% of patients with CRC.3 This mutation results in a constitutively active serine/threonine kinase, leading to alterations in cell growth, cell cycle progression, survival, and migration.4 The BRAF mutations are associated with noncanonical or alternative pathways to CRC tumorigenesis, including the serrated pathway or hypermethylation, and can be present in the setting of microsatellite instability (MSI).5,6 This review discusses the clinical importance of BRAF mutations in CRC, including prognosis and therapeutic strategies.
[caption id="attachment_3328" align="alignleft" width="475"]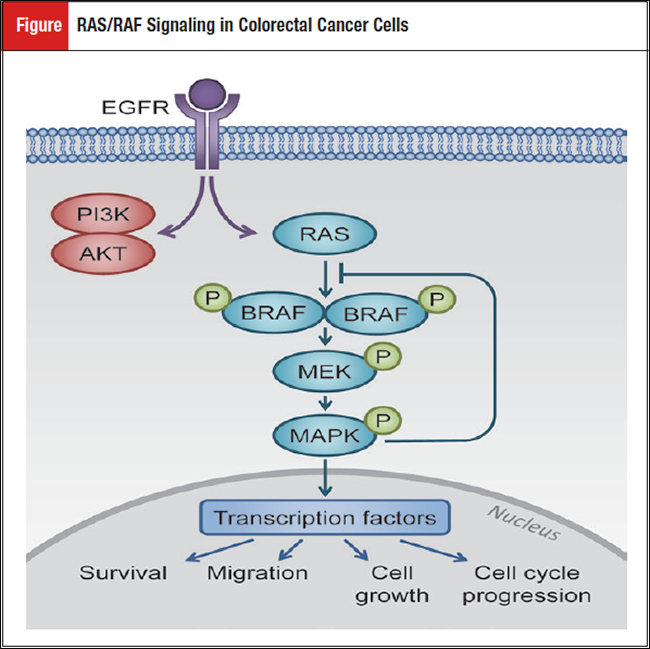
Case Presentation
A 68-year-old female presents with a 4-cm right-sided colon mass after having a colonoscopy for dark stools. Imaging demonstrates multiple unresectable hepatic, pulmonary, and retroperitoneal lymph node metastases. Hepatic biopsy demonstrates a moderate to poorly differentiated adenocarcinoma of the colon. Routine KRAS, NRAS, BRAF, and MSI testing demonstrates that her cancer is BRAF mutated (V600E) and microsatellite stable. KRAS and NRAS are not mutated. She presents to clinic to discuss her prognosis and therapeutic options.
Prognostic Role of BRAF Mutational Status
BRAF mutations in CRC are associated with a worse prognosis in both locoregional and metastatic settings.7-9 BRAF mutations are associated with right-sided malignancies, higher grade, old age, female gender, and higher T and N stages.5 In the first-line setting, BRAF-mutant patients have significantly inferior overall survival (OS) (10.8 vs 16.4 months).10 Following first-line progression, BRAF-mutant patients have a marked reduction in survival compared with patients with wild-type disease (4.2 vs 9.2 months).10
Additionally, BRAF-mutant cancers exhibit a high level of MSI, although not identified in the case above. MSI-high cancers possess alterations in mismatch repair (MMR) leading to very high mutation rates.6 MMR-deficient cancers have been shown to have a better prognosis in CRC.11 Interestingly, the deleterious effect of the BRAF mutation is more pronounced in patients without MSI.5 An analysis by Lochhead et al demonstrated a 5-year CRC-specific survival of 46% for MSI-low/BRAF-mutant patients versus 73% for MSI-high/BRAF-mutant patients.5
Current Therapeutic Strategies
First-Line Chemotherapy
Cancers with BRAF mutations are less likely to respond to first-line treatment (34.6% vs 46.9% in BRAF wild type) and have a low probability of receiving second-line treatment (39% vs 60% in BRAF wild type).10
This suggests that BRAF-mutated patients need intensified first-line treatment. The TRIBE study by Cremolini et al compared FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) plus bevacizumab versus FOLFIRI (fluorouracil, leucovorin, and irinotecan) plus bevacizumab in metastatic CRC patients across all molecular subtypes.12 Their analysis included 28 BRAF-mutant patients. Median OS improved to 19.0 months in the FOLFOXIRI plus bevacizumab group compared with 10.7 months in the FOLFIRI plus bevacizumab cohort. Given the low power of this analysis due to the small sample size, this finding was not statistically significant. However, the treatment effect observed on OS did not differ across all molecular subgroups. These results strengthen the role of intensified therapy up front in metastatic CRC regardless of RAS and BRAF molecular subtypes.
[caption id="attachment_3330" align="alignright" width="650"]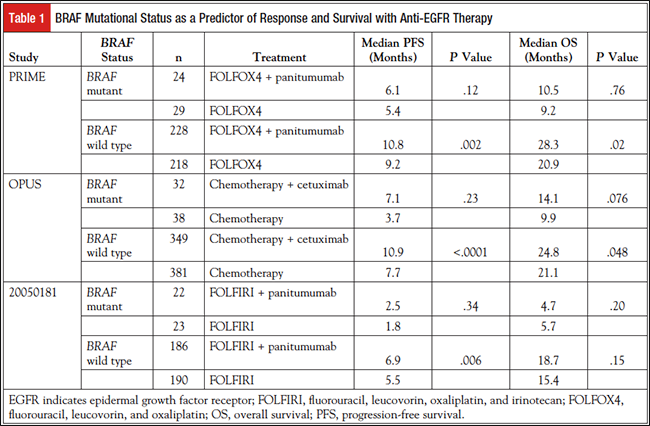
Use of Anti-EGFR Therapy in BRAF-Mutant CRC
The role of monoclonal EGFR-blocking antibodies in BRAF-mutant CRC is not well established. Table 1 summarizes clinical trials involving BRAF-mutant CRC and anti-EGFR therapy generally demonstrating a poor response to treatment.13-15 Given the small sample sizes, it is difficult to detect any meaningful treatment difference with the addition of anti-EGFR therapy. Recently, a meta-analysis by Rowland et al grouped 8 randomized clinical trials that included 351 BRAF-mutant patients.16 This analysis showed that the hazard ratio for OS in patients treated with anti-EGFR therapy (cetuximab or panitumumab) did not depend on BRAF mutational status. The authors concluded that BRAF mutation status is not a negative predictive biomarker for response to anti-EGFR therapy. In contrast, a meta-analysis by Pietrantonio et al concluded that anti- EGFR treatment did not provide any benefit in this subgroup.17 Current National Comprehensive Cancer Network guidelines do not recommend the use of cetuximab and panitumumab in this patient population.18
Targeting BRAF in BRAF-Mutant CRC
BRAF inhibitors that target this pathway include vemurafenib and dabrafenib. Additional drugs being investigated include LGX818 (encorafenib), XL281, and CEP-32496. MEK inhibitors, such as trametinib, have also shown benefit in BRAF-mutant melanoma.19 These inhibitors have a response rate of approximately 50% in melanoma, in which the BRAF mutational rate is more than 60%.20 Unfortunately, use of such inhibitors was found to be ineffective in BRAF-mutant CRC.20-22 Ongoing clinical trials to bypass mechanisms of resistance clinically are listed in Table 2.
[caption id="attachment_3331" align="alignleft" width="650"]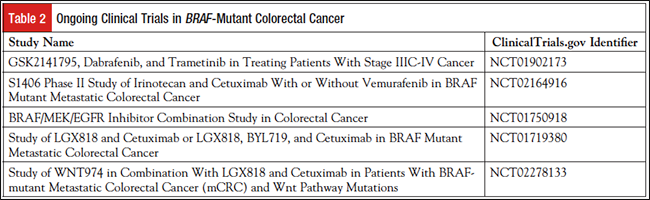
In BRAF-mutant melanoma, improved response rates were observed when BRAF was inhibited in combination with MEK.23 Corcoran et al attempted to suppress the ERK signaling pathway with the combination of dabrafenib plus trametinib.24 Of 43 patients, only 5 achieved a partial response or better, including 1 complete response for more than 36 months. Ten patients remained on study with stable disease for more than 6 months. While on treatment, 9 patients had repeat tumor biopsies that showed downregulation of phosphorylated ERK relative to pretreatment specimens. Given the limited sample size, the study was not able to make any correlation between pharmacodynamic markers and response. Although this study did demonstrate some benefit in a subset of BRAF-mutant CRC, these data suggest that this strategy largely leads to suboptimal MAPK pathway inhibition.
In melanoma, a number of mechanisms have been discovered involving overactivation of the MAPK pathway.25 Unfortunately, resistance mechanisms in melanoma cannot be applied to CRC, as these tumors respond poorly to BRAF inhibitors.2,21 Recent preclinical data have demonstrated that resistance to anti-BRAF medications in CRC is driven by EGFR upregulation to reactivate MAPK signaling.26,27 These data also established that inhibition of EGFR or MEK decreased RAS activation upon treatment with BRAF inhibition in both in vitro and in vivo models.26,27
Yaeger et al completed a study of MAPK signaling inhibition of BRAF and EGFR.28 This pilot trial studied a cohort of 15 patients to assess safety and response rate of vemurafenib with panitumumab. All patients had progressed through at least 1 standard treatment regimen. Tumor regression was seen in 10 of 12 evaluable patients, and 2 patients had stable disease lasting more than 6 months. A low degree of dermatologic toxicity was observed. The most common toxicity was transaminitis. This strategy shows some promise in treating these chemoresistant tumors. However, only a subset of patients responded, and these responses were largely not durable. This suggests the need for further investigation regarding inhibition of downstream proteins, such as ERK, and the role of inhibition of parallel pathways to bypass resistance.
Combination inhibition does ultimately lead to resistance. In a study by Ahronian et al, exon sequencing was performed on tumor biopsies before and after clinical resistance developed to the combination of BRAF, MEK, and EGFR inhibition.29 This demonstrated that resistant tumors can exhibit a range of molecular changes including KRAS mutation, KRAS amplification, BRAF amplification, and MEK1 mutation. The diversity of potential molecular alterations leading to resistance suggests that therapeutic approaches targeting resistance may be difficult to generalize. Additionally, the fact that resistance mechanisms involve reactivation of ERK signaling highlights the critical dependence of BRAF-mutant CRC on sustained ERK signaling to maintain cancer cell proliferation and survival.
Hong et al are conducting an ongoing study regarding the role of anti-BRAF and anti-EGFR combination therapy with chemotherapy.30 In a phase 1b study, 10 patients with advanced and metastatic BRAF-mutated CRC received escalating doses of vemurafenib in combination with cetuximab and irinotecan. Four of the 5 metastatic CRC patients achieved a partial response. Updated data are pending, and a phase 2 trial is recruiting.
Another documented mechanism of resistance involves activation of phosphoinositide 3-kinase (PI3K).31 Preclinical data comparing CRC cell lines with melanoma demonstrate that PI3K is more activated. Additionally, BRAF inhibitor resistance can be overcome with PI3K inhibition. This model has also been confirmed in mouse studies with combinations of anti-BRAF and anti-PI3K/mTOR treatments.32 Synergy was observed with combination of both drugs.
Van Geel et al investigated the role of inhibition PI3K.33 Patients were treated with escalating doses of encorafenib in combination with cetuximab or with escalating doses of encorafenib and BYL719 with cetuximab. Of the 18 patients enrolled, 3 achieved a partial response, including 1 patient in the triple therapy arm. Prolonged disease stability was frequently observed. Updated data are pending.
Conclusion
The BRAF-mutant subtype of CRC possesses a poor prognosis, and improved treatment strategies are clearly needed. Recent advances in the understanding of BRAF biology in CRC are driving current clinical trials in this subset of aggressive cancers. This area of translational research should soon result in improved outcomes for this patient population.
Disclosures
Anita Turk and Dustin Deming have no conflicts of interest to declare.
References
- Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962-972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Tejpar S, Bertagnolli M, Bosman F, et al. Prognostic and predictive biomarkers in resected colon cancer: current status and future perspectives for integrating genomics into biomarker discovery. Oncologist. 2010;15:390-404.
- Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol. 2015;16:281-298.
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-1156.
- Rad R, Cadiñanos J, Rad L, et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell. 2013;24:15-29.
- Yuan ZX, Wang XY, Qin QY, et al. The prognostic role of BRAF mutation in metastatic colorectal cancer receiving anti-EGFR monoclonal antibodies: a meta- analysis. PLoS One. 2013;8:e65995.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-474.
- Sinicrope FA, Yoon HH, Mahoney MR, et al. Overall survival result and outcomes by KRAS, BRAF and DNA mismatch repair in relation to primary tumor site in colon cancers from a randomized trial of adjuvant chemotherapy: NCCTG (Alliance) N0147. J Clin Oncol. 2014;32(suppl). Abstract 3525.
- Seligmann JF, Fisher D, Elliott F, et al. Exploring the poor outcomes of BRAF mutant (BRAF mut) advanced colorectal cancer (aCRC): analysis from 2,530 patients (pts) in randomized clinical trials (RCTs). J Clin Oncol. 2015;33(suppl). Abstract 3509.
- Guastadisegni C, Colafranceschi M, Ottini L, et al. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788-2798.
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306-1315.
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034.
- Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546.
- Peeters M, Oliner KS, Price TJ, et al. Updated analysis of KRAS/NRAS and BRAF mutations in study 20050181 of panitumumab (pmab) plus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC). J Clin Oncol. 2014;32(suppl). Abstract 3568.
- Rowland A, Dias MM, Wiese MD, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112:1888-1894.
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-594.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 2.2016. www.nccn.org/profes sionals/physician_gls/pdf/colon.pdf. Accessed October 26, 2015.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726-736.
- Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28(suppl). Abstract 3534.
- Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032-4038.
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444-451.
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023-4031.
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80-93.
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100-103.
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227-235.
- Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313-1320.
- Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 2015;5:358-367.
- Hong DS, Morris VK, Fu S, et al. Phase 1B study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated advanced cancers and metastatic colorectal cancer. J Clin Oncol. 2014;32(suppl). Abstract 3516.
- Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19:657-667.
- Coffee EM, Faber AC, Roper J, et al. Concomitant BRAF and PI3K/mTOR blockade is required for effective treatment of BRAF(V600E) colorectal cancer. Clin Cancer Res. 2013;19:2688-2698.
- Van Geel R, Elez E, Bendell JC, et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer. J Clin Oncol. 2014;32(suppl). Abstract 3514.

