According to the American Cancer Society, colorectal cancer is the third most common cancer in men and women, and the second leading cause of cancer deaths in the United States.1 An estimated 132,700 new cases of, and 49,700 deaths from, colorectal cancer are expected to occur in 2015. For patients with colorectal cancer, the 5- and 10-year relative survival rates are 65% and 58%, respectively. The 5-year survival rate increases to 90% when colorectal cancer is detected at a localized stage; however, only 40% of colorectal cancers are diagnosed at this early stage. The 5-year survival rate drops to 71% if by the time of diagnosis, the cancer has spread regionally and involves nearby organs or lymph nodes. If the disease has metastasized, the 5-year survival rate decreases to 13%.
Colorectal cancer is one of the major cancer types with existing US Food and Drug Administration (FDA)-approved targeted therapies (Table 1).2-6 These agents are added to standard first-line chemotherapy for metastatic colon cancer, which can consist of 1 of 2 regimens: fluorouracil (Efudex), leucovorin (Wellcovorin), and oxaliplatin (Eloxatin; FOLFOX), or fluorouracil, leucovorin, and irinotecan (Camptosar; FOLFIRI).
Immune Checkpoint Inhibition
Of the many approaches currently under investigation to improve antitumor immune responses in patients with colorectal cancer, immune checkpoint inhibition—targeting the programmed death receptor-1 (PD-1) or programmed death ligand-1 (PD-L1)—has proven to be the most effective thus far in a small, phase 2 study.7 Two anti–PD-1 agents (pembrolizumab [Keytruda] and nivolumab [Opdivo]) are currently FDA approved for the treatment of patients with cancer (Table 2).8,9
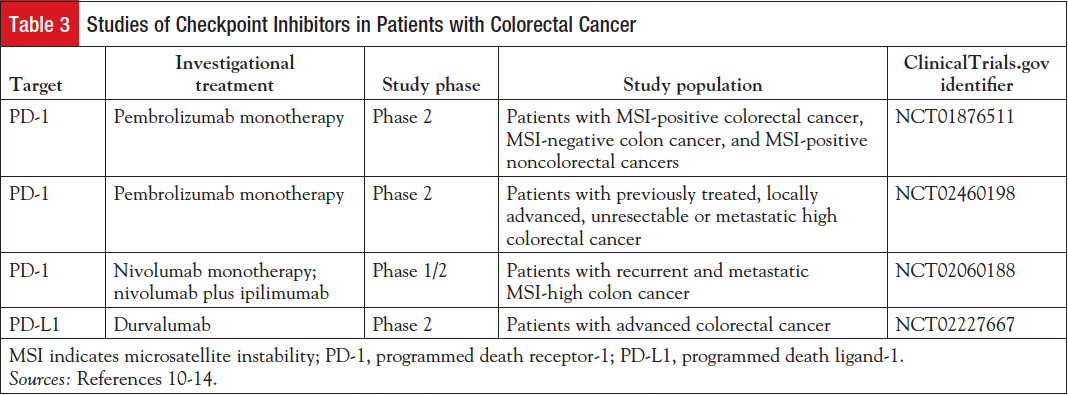
Table 3 lists several clinical studies of checkpoint inhibitor use in patients with colorectal cancer.10-14
Pembrolizumab is being evaluated as monotherapy in patients with colorectal cancer.10,11 Nivolumab is under investigation as monotherapy and in combination with ipilimumab (Yervoy), an antibody that targets cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), in patients with colorectal cancer.12,15 Durvalumab (MEDI4736), a human monoclonal antibody that blocks PD-L1 binding to PD-1 and CD-80 with high affinity and selectivity, is also being evaluated in a phase 2 study of patients with colon cancer.13,14
Microsatellite Instability and DNA Mismatch Repair
Microsatellite instability (MSI) is the expansion or reduction in the length of repetitive DNA sequences (known as microsatellites) in the tumor versus normal DNA.16,17 MSI results from impaired DNA mismatch repair (MMR), a process the human body uses to recognize and repair genetic mismatches generated during DNA replication; a defective MMR system allows mismatch mutations to persist.17 MSI testing can detect an abnormal number of microsatellite repeats, which indicates that the cancer arose from cells with defective MMR genes. A result of “MSI-high” means that a high number of microsatellite repeats were found. A subset of approximately 15% of colorectal cancers has the MSI-high phenotype and a high level of DNA MMR deficiency.18 A better prognosis has been seen in patients with MSI-high colorectal cancers compared with their microsatellite-stable counterparts.
Early studies of checkpoint inhibitors in patients with advanced colorectal cancer were inconclusive; one explanation for these findings is that cancers with a higher number of somatic mutations may stimulate a greater immune response, and, potentially, a higher antigen burden.19,20 In 2013, a study by Raoul A. Droeser, Department of Surgery, University Hospital of Basel, Switzerland, and colleagues demonstrated an association between PD-L1 expression with improved survival in MMR-proficient colorectal tumor specimens.21 Dung Thi Le, MD, Assistant Professor of Oncology, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, and colleagues conducted a phase 2 study based on their hypothesis that tumors with a large number of somatic mutations caused by MMR defects may be vulnerable to immune checkpoint blockade with pembrolizumab.22
Pembrolizumab in Colorectal Cancer and Other Solid Tumors with or without DNA Mismatch Repair
To test whether MSI-high tumors are more responsive to blockade of the PD-1 pathway than microsatellite-stable tumors, Le and colleagues evaluated 41 evaluable, heavily pretreated patients with advanced colorectal cancer and other solid tumors (with and without MMR deficiency) who were treated with pembrolizumab.22 Two cohorts of patients had advanced chemotherapy-refractory colorectal cancer with MSI-high (ie, MMR-deficient) or microsatellite-stable (ie, MMR-proficient) tumors. A third cohort included patients with various cancers that tested MSI-high.
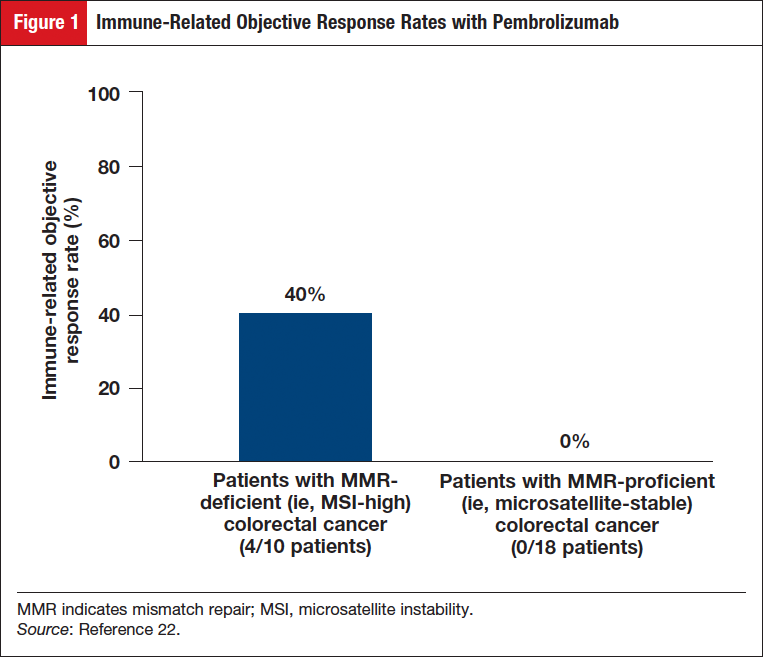
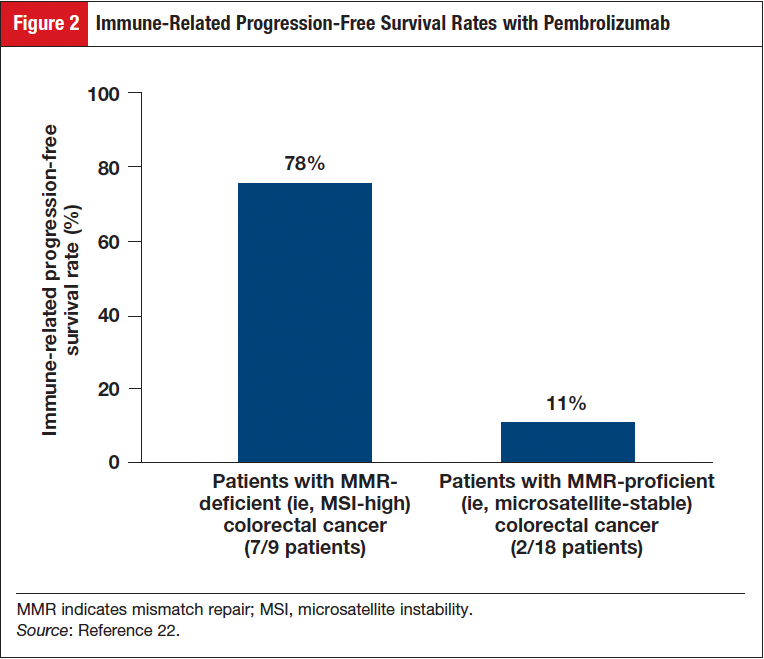
In the group of patients with MMR-deficient colorectal cancer, an immune-related objective response rate (ORR) of 40% (4/10 patients) was observed; no responses (0/18 patients) were observed in the group with MMR-proficient colorectal cancer (Figure 1).22 The immune-related progression-free survival (PFS) rate was 78% (7/9) for patients with MMR-deficient colorectal cancers, and was 11% (2/18) for patients with MMR-proficient colorectal cancers (Figure 2).22
In the cohort with MMR-deficient colorectal cancer, the median PFS and overall survival were not reached, but were 2.2 months and 5.0 months, respectively, in the cohort with MMR-proficient colorectal cancer (hazard ratio for disease progression or death, 0.10 [P <.001], and hazard ratio for death, 0.22 [P = .05]). Similar responses were seen in patients with MMR-deficient noncolorectal and colorectal cancers (immune-related ORR, 71% [5/7 patients]; immune-related PFS rate, 67% [4/6 patients]). It was revealed, via whole-exome sequencing, that a mean of 1782 somatic mutations existed in MMR-deficient tumors versus 73 in MMR-proficient tumors (P = .007). High somatic mutation loads were linked to prolonged PFS (P = .02). Results of the study by Le and colleagues study showed that MMR status predicted the clinical benefit of immune checkpoint blockade with pembrolizumab.22 Based on the encouraging results of this small, early study, a phase 2 study is under way to evaluate the efficacy and safety of pembrolizumab based on MMR status in patients with locally advanced unresectable or metastatic (stage IV) colorectal cancers.11
Nivolumab plus Ipilimumab in Advanced Colorectal Cancer
Studies have demonstrated that combining checkpoint inhibitors with different mechanisms of action shows promise in patients with advanced cancers.23 Nivolumab is under investigation in combination with ipilimumab, an antibody that targets CTLA-4, in a phase 1/2 study of patients with recurrent and metastatic MSI-high colon cancer.12
Durvalumab in Advanced Colorectal Cancer
Durvalumab is a human monoclonal antibody that blocks PD-L1 binding to PD-1 and CD-80 with high affinity and selectivity.13,14 A phase 2 study of this drug is currently under way in patients with advanced colorectal cancer.
Conclusion
Because the prognosis for patients with recurrent or metastatic colorectal cancer is very poor, alternative strategies such as immunotherapy are needed to improve outcomes for patients with advanced disease. Although results from a small study of pembrolizumab indicate that MMR status predicted clinical benefit, additional studies of other agents may uncover additional subsets of patients who may benefit from immunotherapy. As researchers gain understanding about the heterogeneity of colorectal tumors, patient stratification based on molecular subtypes holds promise to improve the clinical outcomes.
References
- American Cancer Society. Cancer facts & figures, 2015. www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed December 17, 2015.
- National Cancer Institute. FDA approval for cetuximab. www.cancer.gov/about-cancer/treatment/drugs/fda-cetuximab. Updated July 2, 2013. Accessed December 21, 2015.
- National Cancer Institute. FDA approval for bevacizumab. www.cancer.gov/about-cancer/treatment/drugs/fda-bevacizumab. Updated December 4, 2014. Accessed December 21, 2015.
- National Cancer Institute. FDA approval for panitumumab. www.cancer.gov/about-cancer/treatment/drugs/fda-panitumumab. Updated July 3, 2013. Accessed December 21, 2015.
- National Cancer Institute. FDA approval for ziv-aflibercept. www.cancer.gov/about-cancer/treatment/drugs/fda-ziv-aflibercept. Updated July 3, 2013. Accessed December 21, 2015.
- National Cancer Institute. FDA approval for regorafenib. www.cancer.gov/about-cancer/treatment/drugs/fda-regorafenib. Updated July 3, 2013. Accessed December 21, 2015.
- Jacobs J, Smits E, Lardon F, et al. Immune checkpoint modulation in colorectal cancer: what’s new and what to expect. J Immunol Res. 2015;2015:158038.
- Keytruda [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2015.
- Opdivo [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2015.
- ClinicalTrials.gov. Phase 2 study of MK-3475 in patients with microsatellite unstable (MSI) tumors. https://clinicaltrials.gov/ct2/show/NCT01876511?term=NCT01876511&rank=1. Updated June 9, 2015. Accessed December 21, 2015.
- ClinicalTrials.gov. Study of pembrolizumab (MK-3475) as monotherapy in participants with previously-treated locally advanced unresectable or metastatic colorectal cancer (MK-3475-164/KEYNOTE-164). https://clinicaltrials.gov/ct2/show/NCT02460198?term=NCT02460198&rank=1. Updated November 3, 2015. Accessed December 21, 2015.
- ClinicalTrials.gov. A study of nivolumab and nivolumab plus ipilimumab in recurrent and metastatic colon cancer (CheckMate 142). https://clinicaltrials.gov/ct2/show/NCT02060188?term=NCT02060188&rank=1. Updated December 8, 2015. Accessed December 21, 2015.
- AstraZeneca. MedImmune and Mirati Therapeutics partner on immuno-oncology combination in lung cancer. www.astrazeneca.com/our-company/media-centre/press-releases/2015/medimmune-mirati-therapeutics-immuno-oncology-combination- lung-cancer-05082015.html. Published August 5, 2015. Accessed December 21, 2015.
- ClinicalTrials.gov. Evaluate the efficacy of MEDI4736 in immunological subsets of advanced colorectal cancer. https://clinicaltrials.gov/ct2/show/NCT0222 7667?term=NCT02227667&rank=1. Updated October 28, 2015. Accessed December 21, 2015.
- US Food and Drug Administration. FDA approves Yervoy to reduce the risk of melanoma returning after surgery. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm469944.htm. Updated October 29, 2015. Accessed December 21, 2015.
- Heinimann K. Toward a molecular classification of colorectal cancer: the role of microsatellite instability status. Front Oncol. 2013;3:272.
- Poulogiannis G, Frayling IM, Arends MJ. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 2010;56:167-179.
- Kloor M, Staffa L, Ahadova A, von Knebel Doeberitz M. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. 2014;399:23-31.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465.
- Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233-2242.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133.