Overview
Cytokines are familiar to the oncologist as the glycoprotein messengers of the immune system and beyond. Their role in cancer treatment has been firmly established since the late 1980s, when investigators reported that interleukin (IL)-2 administration plus adoptive transfer of lymphokine-activated killer cells were effective treatment for refractory solid tumors.1 IL-2 has proven useful for the treatment of advanced renal cell carcinoma (RCC) and metastatic melanoma.2,3 It was FDA approved as aldesleukin (Proleukin) for the treatment of RCC in 1992 and for the treatment of melanoma in 1998.4 Since then, other cytokines such as interferon beta have been approved for the treatment of cancer and chronic autoimmune diseases.5
In the case of IL-2 for the treatment of advanced RCC and metastatic melanoma, the cytokine itself can act as an anticancer agent. Cytokines can also play an important auxiliary role as immunologic adjuvants. It should be clarified that adjuvants are applied differently in immunology than in oncology. In the immunology setting, they are typically given along with immunogens and enhance immune responses; vaccines have adjuvants as part of their composition. In the United States, only aluminum gels or aluminum salts are currently licensed for use as standard vaccine adjuvants.6
For cancer vaccines, such as the bivalent human papillomavirus (HPV) vaccine Cervarix, oil-containing adjuvants such as monophosphoryl lipid A (MPLA) boost innate immunity and build subsequent lasting immunity.7 MPLA is derived from the lipopolysaccharide (LPS) of Salmonella minnesota R595.8 Like all types of LPS, MPLA activates toll-like receptor-4 with potencies similar to that of LPS, as measured by various biological assays, but with less toxicity, presumably because MPLA activates the downstream proinflammatory MyD88 adaptor protein less effectively than does LPS.9,10
Cytokines have the potential to augment immune responses. Depending on the cytokine, specific aspects of immunity can be enhanced from innate immunity through helper T-cell (TH) responses to the generation of cytotoxic T lymphocytes (CTLs). Viral vector vaccines that generate immune responses to the human immunodeficiency virus (HIV) for the prevention of acquired immunodeficiency syndrome have tested several cytokines for their efficacy as adjuvants in enhancing immune responses. Research in primate models showed that IL-2 and IL-4 boosted antibody titers, IL-12 increased CD4+ T-cell and CTL activity, and IL-15 augmented CTL activity and antibody titers.11 These results are all the more striking when one considers that HIV itself mediates immunodeficiency in the host.
As mentioned previously, IL-2 is approved as an anticancer treatment. It was the first cytokine used to treat cancer, originally used as a vaccine adjuvant due to its T-cell enhancing properties. However, IL-2 has many adverse effects, making its widespread use problematic.12 In clinical trials, its immune-enhancing properties were difficult to dissociate from its direct anticancer properties, and thus in most trials vaccines plus IL-2 were no better than IL-2 alone.13 Other cytokines, such as IL-7 and IL-15, might be better adjuvants. IL-7, unlike IL-2, is not produced by lymphocytes and does not support natural killer (NK) cells.12 IL-15 is constitutively expressed by a variety of cell types, but remains membrane-bound, not secreted. It is required for differentiation of NK cells and helps to differentiate and expand CD8+ T cells.12 CD4+ T cells produce homolog IL-21. The number of trials reported at ClinicalTrials.gov for these cytokines (23 trials for IL-21, 40 for IL-15, and 36 for IL-7) indicates the past and present level of interest in using these cytokines in cancer therapy.
An interesting study recently demonstrated the synergistic role of cytokines with antibodies in a HER2/neu mouse tumor model.14 In these experiments, investigators compared challenges with lethal TUBO carcinoma cells in normal BALB/c mice, and in BALB/c mice deficient for Fc gamma or interferon gamma. Normal BALB/c mice given the tumor vaccine were able to survive the tumor challenge, whereas mice unable to mount complete antibody responses (deficient for the Fc gamma) or protective cytokine responses (deficient for interferon gamma) were not.14 These experiments underscore the complex immune responses brought into play when tumors are rejected, and also highlight how different aspects of the immune system interact with each other to protect the host.
In the clinic, cytokine adjuvants are used to create cytokine-induced killer (CIK) cells. In this technique, patient cells are activated by incubation with IL-2 or IL-2 plus interferon gamma, then reinfused into the patient.15 The advantages of CIK cells are the nonmajor histocompatibility lysis of tumor cells with effective cytolytic activity against both hematopoietic and solid tumors.15 In the clinical setting, CIK cells were used to treat triple-negative breast cancer postmastectomy. Of 90 patients enrolled, 45 patients received CIK cells, and results showed that CIK cells resulted in higher overall survival and disease-free survival than treatment without CIK cells.16 Multivariate analysis demonstrated that CIK cells was an independent prognostic factor for overall survival in this cohort of patients with triple-negative breast cancer.16 These results argue strongly for further work into the potential use of cytokines as immunologic adjuvants for cancer treatment.
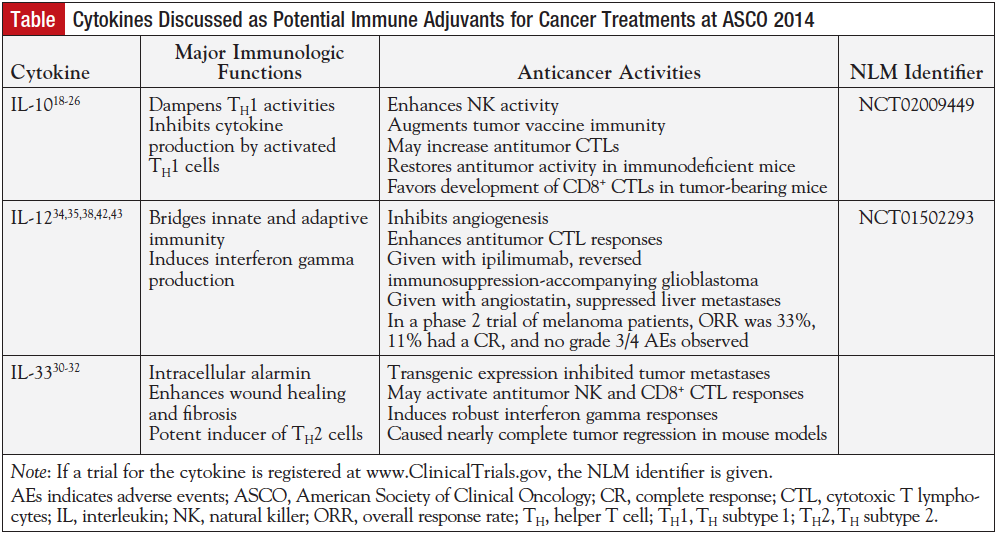
At the recent American Society of Clinical Oncology (ASCO) annual meeting, recent work regarding 3 cytokines as potential cancer vaccine adjuvants was presented. These research highlights are presented as follows, along with a brief background regarding each cytokine.
IL-10
Originally described as a cytokine-suppressing factor with dampening T-cell responses as its major function, IL-10 has since proved to have more nuanced functions vis-à-vis the immune system.17 In certain conditions, IL-10 can be immune-enhancing.18 In severely immunodeficient mice, IL-10 enhanced NK-cell activity.19 In normal mice, IL-10–secreting CD4+ T cells rejected glioma cells.20 Other studies showed that IL-10 given after antitumor vaccination augmented tumor immunity induced by the vaccine.19 Moreover, the number of CTLs in animals treated with the vaccine/IL-10 regimen versus vaccine alone was higher.21 Recent work revealed that mice deficient in IL-10 were unable to mount interferon gamma–dependent tumor surveillance, and there was an overexpression of IL-10 by mice-enhanced tumor suppression. Treating the IL-10–deficient mice with IL-10 conjugated to polyethylene glycol, called pegylated IL-10, restored antitumor responses.22 The authors’ findings showed how complex T-cell polarization is, particularly in the tumor-bearing state when T-cell polarization is dysregulated.23 In the tumor-bearing state, it is possible that exogenous stabilized IL-10 favors development of CD8+ CTLs mediated by interferon gamma, which would aid tumor rejection.22-25 If this hypothesis is correct, then IL-10 should be an excellent candidate for a cancer vaccine adjuvant.
Patients are being enrolled in a phase 1 trial to determine the safety and preliminary efficacy of pegylated IL-10, or AM0010. Patients with various types of solid tumors (melanoma, non–small-cell lung cancer, RCC, colorectal cancer, castrate-resistant prostate cancer, ovarian cancer, and pancreatic cancer) will enroll to self-administer daily doses of AM0010 for 4 cycles over the course of 1 month. At the end of the study, safety, tolerability, and preliminary antitumor activity are graded. The study should be complete by the end of this year. This trial is registered at ClinicalTrials.gov, and the trial number is NCT02009449.26
IL-33
One of the more recently described cytokines, IL-33 is a member of the IL-1 family of cytokines. It is a ligand for the orphan receptors of the IL-1 receptor family called ST2. Constitutively expressed by many cell types such as keratinocytes, fibroblasts, and mucosal epithelial cells, IL-33 functions as an intracellular alarmin, signaling when released due to cell damage. When secreted, IL-33 acts as a cytokine, binding to its receptor and thereby activating downstream signaling events.27 Much of the biology of IL-33 is emerging from studies on its role in wound healing and fibrosis. These studies reveal that IL-33 is a potent inducer of TH2 (TH subtype 2) cells, as these cells are the predominant cell type expressing ST2.28 Furthermore, IL-33 is essential for normal functioning of the gastrointestinal immune system.29 Dysregulation of IL-33 function and abnormal loci of secretion may play a role in the pathogenesis of inflammatory bowel disease.29 Because IL-33 signaling activates the mitogen-activated protein kinase inflammatory signaling pathway, a role for it in fibrotic diseases such as inflammatory pancreatitis is postulated.29
The role of IL-33 in cancer is more difficult to ascribe. Mice deficient in the IL-33 receptor ST2 clear tumor cells more rapidly than wild-type mice do. Its role in maintaining gut function may implicate it as a pathogenic factor in the development of some types of gastrointestinal cancers.27 However, transgenic expression of IL-33 inhibited tumor metastases in melanoma and lung cancer mouse models; this inhibition was abrogated when CD8+ or NK cells were depleted from the mice. The authors suggest that IL-33 plays an important role in activating protective CD8+-mediated and NK-mediated antitumor responses in vivo. In their in vitro studies, they also showed that adding IL-33 to cultures of CTLs or NK cells increased each cell type’s capability to lyse tumor cells.30 Detailed studies of IL-33 as a cancer adjuvant revealed that it induced robust interferon gamma responses without elevating either immunoglobin E or IL-4, in response to a HPV-based vaccine. IL-33 acted as a strong adjuvant when given in conjunction with the vaccine, such that robust antitumor responses were generated, resulting in tumor regression.31
More research on IL-33 as a potent cancer vaccine adjuvant was presented at the 2014 ASCO meeting. In this study, IL-33 was given to human volunteers by electroporation as part of clinical trials for an HPV vaccine, based on previous data demonstrating that 78% to 90% of volunteers generated antigen-specific CD8+ and CD4+ T-cell responses. In mice that received IL-33, vigorous interferon gamma responses were noted; this was not noted for IL-4. CD4+ and CD8+ T-cell responses were enhanced when IL-33 was given compared with antigen alone (24% - 25% vs 18%, and 66% - 67% vs 56%, respectively). Coadministration of IL-33 plus antigen resulted in 100% protection against the HPV challenge. Depending upon which of 2 isoforms of IL-33 was given, 9 of 10 or 10 of 10 mice had complete tumor regression. The authors note that these data support their hypothesis that IL-33 can serve as a potent adjuvant for enhancing immune responses to cancer vaccines. The IL-33 adjuvant is not yet in clinical trials; however, results of such trials are eagerly awaited.32
IL-12
IL-12 is an unusual cytokine in that it is a heterodimer bearing part of its receptor as 1 of its 2 chains. It enhances T-cell function and is a major factor for TH1 (TH subtype 1) cell differentiation. Furthermore, it serves as the bridge between innate and adaptive immunity.33 IL-12 induces interferon gamma, and through this cytokine inhibits angiogenesis.34 Moreover, IL-12 enhances antitumor CTL responses in an antigen-specific manner, most likely due to its differentiation of TH1 cells.35
In an early mouse study, adenocarcinoma cells engineered to express IL-12 or IL-12 as recombinant protein were injected into host mice. Mice with the tumor treated with tumor cells expressing IL-12 had high tumor regression rates, compared with mice without the tumor. Exogenous administration of IL-12 to mice with tumors resulted in the highest tumor regression rate of all groups.36 Female mice expressing the HER2/neu oncogene and treated with exogenous IL-12 showed delayed onset of tumorigenesis. In the tumors that developed, there was an increase in immune cells in the tumors, reduced tumor microvessel formation, and a high amount of hemorrhagic necrosis in the tumors.37 Combined with anti–CTLA-4 (cytotoxic T-lymphocyte antigen-4) blockade such as ipilimumab, IL-12 reversed the immunosuppressive environment that accompanies glioblastoma. Specifically, this combination reduced the number of FoxP3+ regulatory T cells and increased the number of effector T cells.38 These findings imply that IL-12 may play a role as an adjuvant in cancer treatment.
Using a virus to fight cancer is a novel approach; one such approach was described at ASCO for other types of cancer. Investigators designed an oncolytic herpes virus vector carrying the immune-stimulating IL-12, and compared it with the same vector without IL-12.39 Adding IL-12 to the oncolytic herpes virus vector (called by the authors an armed vector) enhanced antitumor activity in glioblastoma models compared with the unarmed vector. The IL-12–modified vector prolonged survival, inhibited angiogenesis, and effected tumor regression in vivo, especially when another vector armed with angiostatin was added.40 Part of the body’s angiogenesis-regulating mechanism, angiostatin has been investigated for use in cancer treatment.41 It was shown previously that long-term expression of angiostatin by an adenoviral vector suppressed liver metastases in a mouse model with little toxicity.42 Antitumor activity of the IL-12–expressing herpes viral vectors was assessed in vitro and in vivo. In a cervical cancer model, both vectors were highly cytotoxic and both had antitumor effects in vivo.39 Mice given the herpes viral vector had an increased infiltrate of CD8+ cells in their spleen, compared with control mice receiving no vector.39 These results are very preliminary; however, this approach is one that may be applied to other types of cancer, and for which new results are expected in the near future.
In an ongoing phase 2 clinical trial that will test an IL-12–expressing vector via intratumoral delivery, 29 advanced melanoma patients are enrolled thus far.43 The vector, delivered by electroporation, is a plasmid rather than a viral vector. Preliminary results show that the overall response rate is 33%, with 11% having a complete response. Tumor regression was observed in 62% of patients.43 The most common adverse events (all grade 1/2; no grade 3/4 were observed) were transient pain and inflammation at the treatment site.43 Early analysis revealed a marked increase (about 100%) of intratumor NK cells.43 Based on these results, an expansion is planned. Full trial information is available at Clinical Trials.gov, and the trial number is NCT01502293.
Conclusions
Cytokines, in particular those discussed in this review, have an important place in cancer treatment. The cytokines discussed at ASCO 2014 are summarized and their immune adjuvant properties compared in the Table. Whether a primary treatment, as is the case for IL-2, or as immune adjuvants in the future, cytokines will continue to be a focus for future therapy. It will be exciting to see the results of phase 3 trials of IL-33, IL-12, and IL-10.
References
- Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889-897.
- National Cancer Institute website. Melanoma Treatment: PDQ. Treatment Option Overview for Melanoma. Last modified February 21, 2014. www.cancer.gov/cancertopics/pdq/treatment/melanoma/HealthProfessional/page4#Section_859. Accessed July 10, 2014.
- National Cancer Institute website. Renal Cell Cancer Treatment: PDQ. Last modified February 21, 2014. www.cancer.gov/cancertopics/pdq/treatment/renalcell/HealthProfessional/page8#Section_179. Accessed July 10, 2014.
- US Food and Drug Administration. Aldesleukin Product Approval Information - Licensing Action. 2010. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugs areDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplica tions/ucm080733.htm. Accessed July 11, 2014.
- US Food and Drug Administration. Therapeutic Biological Products Approvals. 2003. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandap proved/approvalapplications/therapeuticbiologicapplications/ucm080402.htm. Accessed July 11, 2014.
- Centers for Disease Control and Prevention. Frequently Asked Questions about Adjuvants. 2010. www.cdc.gov/vaccinesafety/concerns/adjuvants.html.
- Dubensky TW Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010; 22:155-161.
- Ulrich JT, Myers KR. Monophosphoryl Lipid A as an Adjuvant. In: Powell M, Newman M, eds. Vaccine Design. New York, NY: Springer US; 1995:495-524.
- Mata-Haro V, Cekic C, Martin M, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628-1632.
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231-3240.
- Morrow MP, Weiner DB. Cytokines as adjuvants for improving anti-HIV responses. AIDS. 2008;22:333-338.
- Capitini CM, Fry TJ, Mackall CL. Cytokines as adjuvants for vaccine and cellular therapies for cancer. Am J Immunol. 2009;5:65-83.
- Chapman PB. Combining a peptide vaccine with high-dose interleukin-2. J Clin Oncol. 2008;26:2250-2251.
- Curcio C, Di Carlo E, Clynes R, et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her-2/neu carcinomas. J Clin Invest. 2003;111:1161-1170.
- Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11:83.
- Pan K, Guan X-X, Li Y-Q, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014;20:3003-3011.
- Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765.
- Mocellin S, Marincola FM, Riccardo Rossi C, et al. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61-76.
- Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Bio. 2005;78:1043-1051.
- Segal BM, Glass DD, Shevach EM. IL-10-producing CD4+ T cells mediate tumor rejection. J Immunol. 2002;168:1-4.
- Fujii S, Shimizu K, Shimizu T, et al. Interleukin-10 promotes the maintenance of antitumor CD8+ T-cell effector function in situ. Blood. 2001;98:2143-2151.
- Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFN?-dependent tumor immune surveillance. Cancer Cell. 2011;20:781-796.
- Teng MWL, Darcy PK, Smyth MJ. Stable IL-10: a new therapeutic that promotes tumor immunity. Cancer Cell. 2011;20:691-693.
- Oft M. IL-10: Master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194-199.
- Mumm JB, Oft M. Pegylated IL-10 induces cancer immunity: the surprising role of IL-10 as a potent inducer of IFN-?-mediated CD8+ T cell cytotoxicity. Bioessays. 2013;35:623-631.
- Bauer TM, Papadopoulos KP, Autio KA, et al. A first-in-human study of pegylated recombinant human IL-10 (AM0010), daily administered for four months in selected advanced solid tumors. J Clin Oncol. 2014;32(5S):Abstract TPS3126.
- Jovanovic IP, Pejnovic NN, Radosavljevic GD, et al. IL-33/ST2 axis in innate and acquired immunity to tumors. Oncoimmunology. 2012;1:229-631.
- Chackerian AA, Oldham ER, Murphy EE, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551-255.
- Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5:18.
- Gao K, Li X, Zhang L, Bai L, et al. Transgenic expression of IL-33 activates CD8+ T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463-471.
- Villarreal DO, Wise MC, Walters JN, et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014;74:
1789-1800.
- Villarreal D, Wise M, Walters J, et al. Synthetic IL-33 DNA as anti-tumor adjuvant in vivo. J Clin Oncol. 2014;32(5S):Abstract 3098.
- Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677-4685.
- Dias S, Boyd R, Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int J Cancer. 1998;78:361-365.
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155-168.
- Cavallo F, Giovarelli M, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049-1058.
- Boggio K, Nicoletti G, Di Carlo E, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinoma in two lines of her-2/neu transgenic mice. J Exp Med. 1998;188:589-596.
- Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell–mediated glioma rejection. J Exp Med. 2013;210: 2803-2811.
- Kagabu M, Miura Y, Saito T, et al. Impact of new oncolytic herpes simplex virus vector armed with interleukine-12 for cervical cancer therapy. J Clin Oncol. 2014;32 (5S):Abstract 3102.
- Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15:591-599.
- Dell’Eva R, Pfeffer U, Indraccolo S, et al. Inhibition of tumor angiogenesis by angiostatin: from recombinant protein to gene therapy. Endothelium. 2002;91:3-10.
- Xu R, Sun X, Tse L-Y, et al. Long-term expression of angiostatin suppresses metastatic liver cancer in mice. Hepatology. 2003;37:1451-1460.
- Daud A, Algazi AP, Ashworth MT, et al. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. J Clin Oncol. 2014;32(5S):Abstract 9025.

