OPDIVO® (nivolumab) in Combination with Chemotherapy as First-Line Therapy in Patients with Advanced or Metastatic Gastric Cancer, Gastroesophageal Cancer, and Esophageal Adenocarcinoma as well as Unresectable Advanced or Metastatic Esophageal Squamous-Cell Carcinoma: Results from Checkmate 649 and Checkmate 648
INTRODUCTION
In 2022, approximately 47,000 people in the United States were diagnosed with gastric cancer (GC), gastroesophageal junction cancer (GEJC), and esophageal adenocarcinoma (EAC), with approximately 27,500 deaths resulting from these cancer types.1,2 Men are more likely to be affected by these malignancies, with 60% of GC cases and 80% of GEJC and EAC cases affecting men.1,2 Adenocarcinoma is the most common type of cancer of the esophagus among white patients, whereas esophageal squamous-cell carcinoma (ESCC), the second most common type, is more common in African American patients.1
There are significant unmet needs in patients with these malignancies. Treatment options for patients with metastatic GC are limited, and these patients often have a poor prognosis.3 More than half of new cases are unresectable, with a median overall survival (OS) of <1 year for patients treated with chemotherapy alone.4,5
Recently, the US Food and Drug Administration (FDA) approved OPDIVO® (nivolumab), in combination with fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of adult patients with advanced GC, GEJC, and EAC based on the results of the phase 3 Checkmate 649 clinical trial in which OPDIVO plus chemotherapy demonstrated superior OS versus chemotherapy alone in these patients.6
Moreover, OPDIVO, in combination with fluoropyrimidine- and platinum-containing chemotherapy, has also been FDA-approved for the first-line treatment of adult patients with unresectable advanced or metastatic ESCC based on the results of the phase 3 Checkmate 648 clinical trial in which OPDIVO plus chemotherapy demonstrated superior OS versus chemotherapy alone in these patients.7,8
Based on these 2 large, phase 3, international trials, OPDIVO plus chemotherapy has been FDA-approved as first-line therapy for advanced or metastatic upper gastrointestinal cancer regardless of tumor location, histology, or programmed cell death ligand 1 (PD-L1) status. Key results from these 2 pivotal clinical trials will be reviewed in this publication.
Checkmate 649
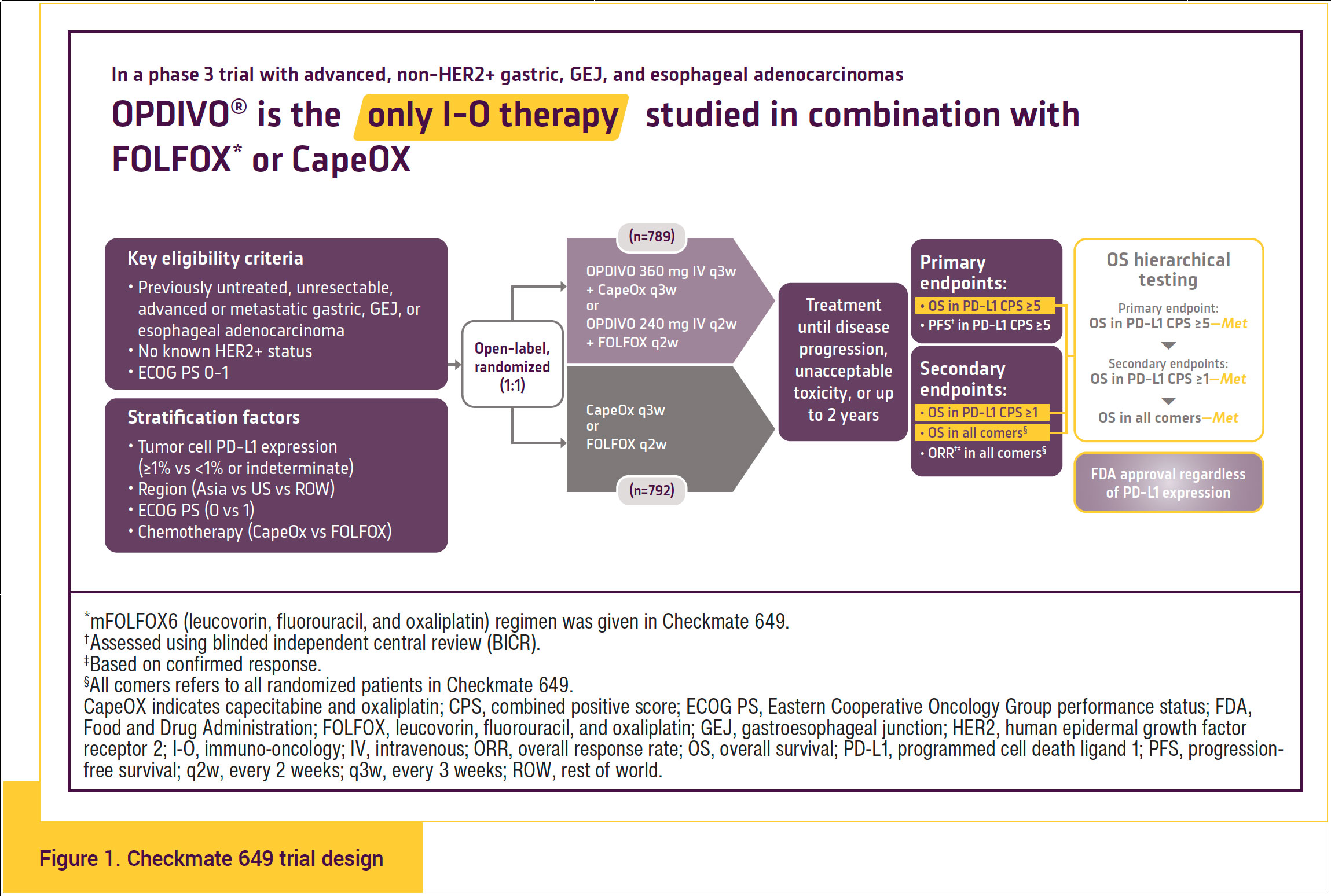
Checkmate 649 was a multicenter, randomized, phase 3, open-label trial that evaluated OPDIVO plus capecitabine and oxaliplatin (CapeOX) or OPDIVO plus fluorouracil, leucovorin, and oxaliplatin (FOLFOX) compared with CapeOX or FOLFOX alone as first-line therapy in patients with previously untreated, HER2-negative, unresectable, advanced or metastatic GC, GEJC, or EAC and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.9,10 Further stratification factors were tumor cell PD-L1 expression of ≥1% versus <1% or indeterminate; geographic region (Asia vs United States/Canada vs the rest of the world); ECOG performance status 0 versus 1; and chemotherapy consisting of either CapeOX or FOLFOX. Patients in the trial were randomized 1:1 to receive OPDIVO plus chemotherapy or chemotherapy alone.9,10 The clinical trial evaluated the following treatment regimens: 240 mg of OPDIVO with FOLFOX or FOLFOX alone administered every 2 weeks, or 360 mg of OPDIVO with CapeOX or CapeOX alone administered every 3 weeks.6
Patients with known HER2-positive status, untreated central nervous system metastases, peripheral neuropathy, autoimmune disease, hepatitis B or hepatitis C virus, and human immunodeficiency virus or known acquired immunodeficiency syndrome were excluded from Checkmate 649 (Figure 1).11
The primary tumor location in Checkmate 649 was approximately 70% as GC, 17% as GEJC, and 14% as EAC, with 38% to 40% of the patients having liver metastases.
OPDIVO in combination with fluoropyrimidine- and platinum-containing chemotherapy is indicated for the treatment of patients with advanced or metastatic GC, GEJC, and EAC.
- In Checkmate 649, in the OPDIVO plus chemotherapy arm, patients who discontinued chemotherapy were permitted to receive OPDIVO monotherapy at 240 mg every 2 weeks, 360 mg every 3 weeks, or 480 mg every 4 weeks up to 2 years after treatment initiation.11
- The trial excluded patients with known HER2-positive status or who had untreated central nervous system metastases.11
- Tumor specimens were evaluated prospectively using the PD-L1 IHC 28-8 pharmDx assay at a central laboratory.11
- The efficacy analysis in patients with PD-L1 combined positive score (CPS) ≥5 included 473 patients in the OPDIVO plus FOLFOX or CapeOX arm and 482 patients in the FOLFOX or CapeOX arm.12
- Minimum follow-up time was 36 months.12
The results of Checkmate 649 were updated at the 2023 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium with expanded analyses of efficacy and safety at a 36-month follow-up.12
At a prespecified primary analysis with a minimum follow-up of 12.1 months, OPDIVO plus chemotherapy resulted in significant improvements in OS and progression-free survival (PFS) compared with chemotherapy alone in all comers, including PD-L1 expressors and non-expressors. Additional 12-month follow-up from the primary analysis demonstrated that OPDIVO plus chemotherapy continued to show improvement in OS compared with chemotherapy alone.
Overall, these data demonstrate a sustained benefit with a longer follow-up period.
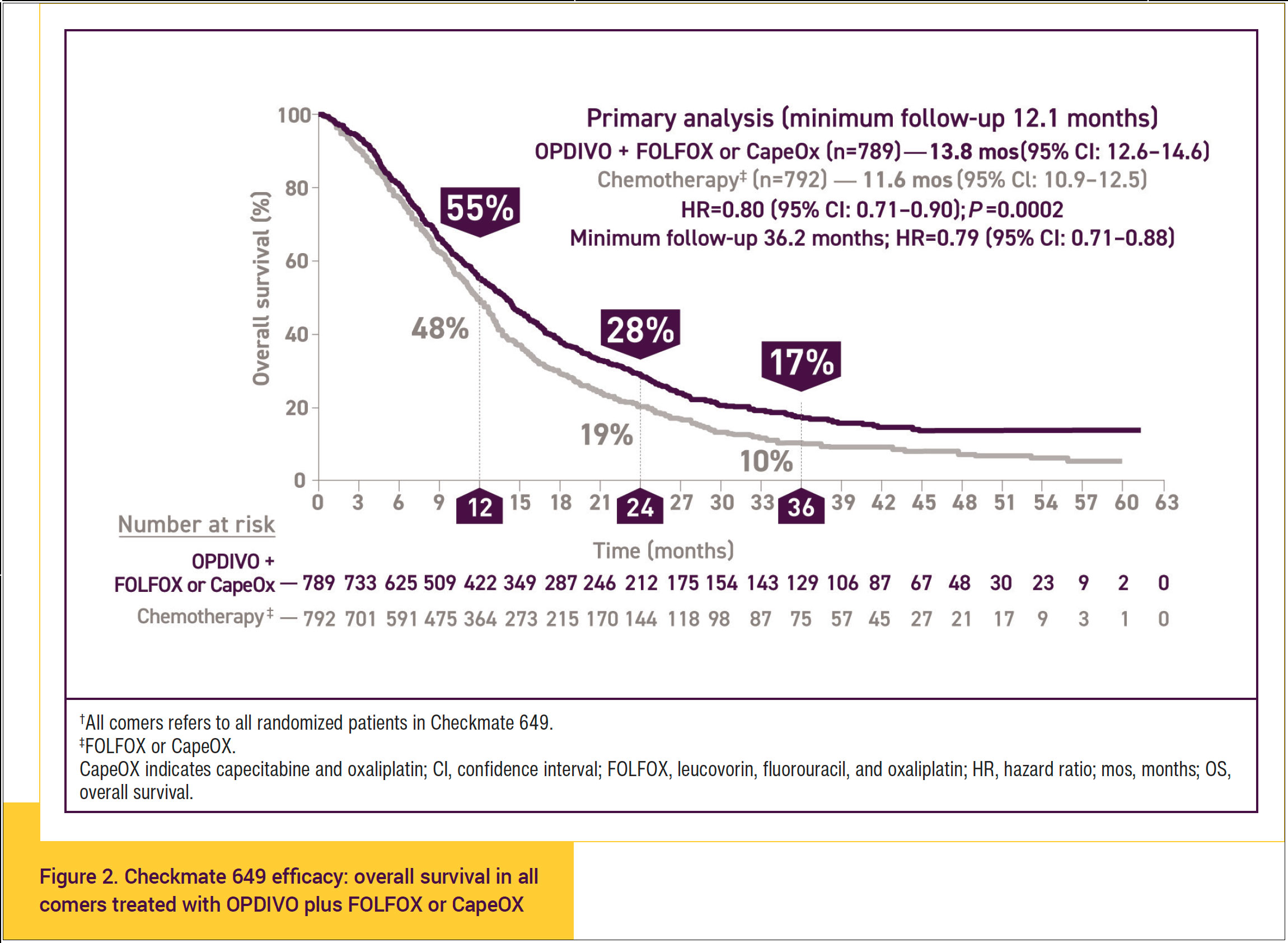
- OS of 17% in all comers at 36 months with treatment with OPDIVO plus chemotherapy versus 10% OS at 36 months with chemotherapy alone is significant. This sustained survival improvement with OPDIVO plus chemotherapy is meaningful for patients in terms of being able to live longer, even with stage IV disease (Figure 2).11-13
- At the extended follow-up at 3 years, a significant difference in median OS of 13.8 months was observed for all comers (95% confidence interval [CI], 12.6-14.6) with OPDIVO plus FOLFOX or CapeOX versus 11.6 months (95% CI, 10.9-12.5) for chemotherapy alone (hazard ratio [HR], 0.80; 95% CI, 0.71-0.90; P = .0002).12
- A significant difference in median PFS of 7.7 months was observed for all comers (95% CI, 7.1-8.6) with OPDIVO plus FOLFOX or CapeOX versus 6.9 months (95% CI, 6.7-7.2) for chemotherapy alone (HR, 0.79; 95% CI, 0.70-0.89).12,13
Thus, the 3-year follow-up shows persistent improvement in both OS and PFS, and it shows that the clinical improvement is durable in people who are getting OPDIVO plus FOLFOX or CapeOX in this setting.
At the 36-month extended analysis, 47% of all comers receiving OPDIVO plus chemotherapy responded and 10% were complete responders, compared with a 37% response rate and 7% complete responders in those receiving chemotherapy alone.
The proportion of patients with an objective response and those with a complete response was numerically higher with OPDIVO plus chemotherapy compared with chemotherapy alone in all randomized patients. Moreover, the median duration of response was 8.5 months (95% CI, 7.2-9.9) with OPDIVO plus chemotherapy compared with 6.9 months (95% CI, 5.8-7.2) with chemotherapy alone.13,14
These data show that there is an increased benefit in overall response rate and complete response rate with OPDIVO plus chemotherapy compared with chemotherapy alone.
The efficacy end points from the 36-month follow-up are clinically meaningful and relevant to convey to all patients when considering treatment.
The data show that therapy with OPDIVO plus chemotherapy improves OS. Cancer shrinkage is faster and better with OPDIVO plus chemotherapy. If you have bulky disease and chemotherapy alone is not optimal, OPDIVO plus chemotherapy is shown to improve the response rate, as well as make it possible for patients to live longer.
In the 3-year follow-up, the data show that the overall response rate, PFS, and OS are better in the OPDIVO plus chemotherapy arm than with chemotherapy alone.
In Checkmate 649, the results demonstrated a significant survival advantage with OPDIVO plus chemotherapy for each end point at 3 years’ follow-up, including in PD-L1 expressors and non-expressors. These data suggest that OPDIVO plus chemotherapy represents the new standard in first-line treatment in all patients with advanced GC/GEJC/EAC, even in those who are PD-L1 non-expressors.
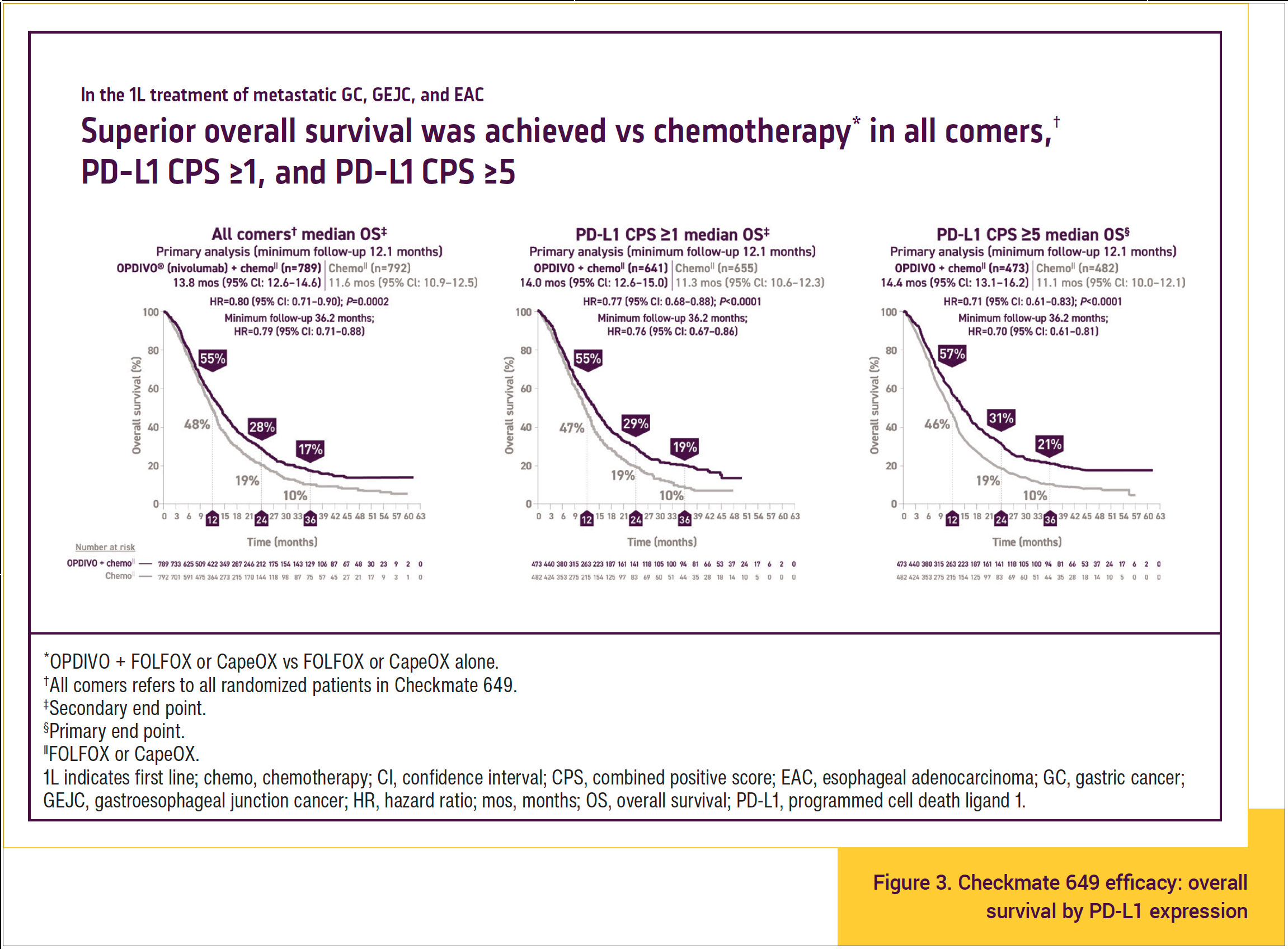
OS consistently favored OPDIVO plus chemotherapy compared with chemotherapy alone across multiple prespecified baseline demographics and disease characteristics in the primary population and all randomized patients. Particularly, survival benefit with OPDIVO plus chemotherapy occurred in all comers regardless of PD-L1 expression (Figure 3).10,11,13-16 At 3 years of follow-up, superior OS was seen in patients treated with OPDIVO plus chemotherapy compared with chemotherapy alone in all comers.
- In patients with PD-L1 CPS ≥1, median OS was 19% in those treated with OPDIVO plus chemotherapy compared with 10% in those treated with chemotherapy alone at 36 months.
- In patients with PD-L1 CPS ≥5, median OS was 21% in those treated with OPDIVO plus chemotherapy compared with 10% in those treated with chemotherapy alone at 36 months.
The important point is that all patients, regardless of PD-L1 score, had greater survival benefit with OPDIVO plus chemotherapy than with chemotherapy alone, so OPDIVO plus chemotherapy can be used for all comers with advanced gastric, gastroesophageal junction, or esophageal cancer.
In an exploratory analysis at 36 months in Checkmate 649, complete response rates were numerically higher in patients with low or no PD-L1 expression who were treated with OPDIVO plus chemotherapy compared with chemotherapy alone.
In patients with PD-L1 CPS <1, there was a 7.9% complete response rate in the group treated with OPDIVO plus chemotherapy compared with 6.4% in the group treated with chemotherapy alone. In patients with PD-L1 CPS <5, there was a 7.1% complete response rate in the group treated with OPDIVO plus chemotherapy compared with 5.4% in the group treated with chemotherapy alone.14 This suggests a benefit of OPDIVO plus chemotherapy compared with chemotherapy alone in all comers regardless of PD-L1 score with respect to achieving a complete response.
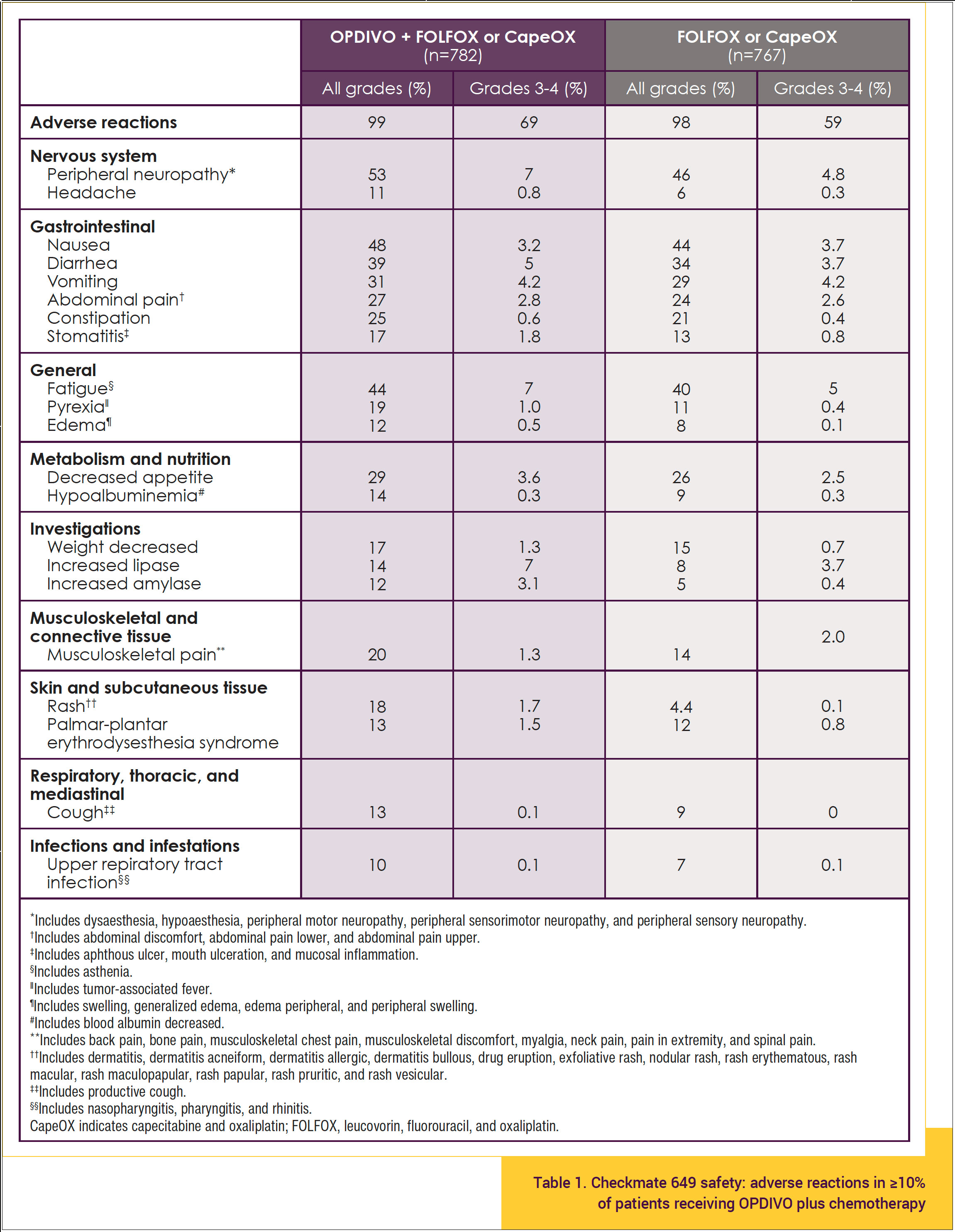
In Checkmate 649, OPDIVO and/or chemotherapy was discontinued in 44% of patients and at least 1 dose was withheld in 76% of patients because of an adverse reaction. Serious adverse reactions occurred in 52% of patients treated with OPDIVO in combination with chemotherapy. Table 1 shows the incidence of adverse reactions in ≥10% of patients receiving OPDIVO plus FOLFOX or CapeOX.11
The safety profile of OPDIVO plus chemotherapy shown in Table 1 is consistent with the known safety profiles of the individual treatments, and no new safety signals were identified. The incidence of treatment-related adverse events (TRAEs) at the 36-month follow-up analysis was similar to the primary analysis at 12.1 months. Moreover, grade 3 to 4 TRAEs with potential immunologic etiology occurred in ≤5% of patients, and the overall safety profile was acceptable.
In April 2021, OPDIVO, in combination with fluoropyrimidine- and platinum-containing chemotherapy, was approved by the FDA for the treatment of patients with advanced or metastatic gastric, gastroesophageal junction, or esophageal cancer in all comers (both PD-L1 expressors and non-expressors) based on the phase 3 Checkmate 649 trial.6
Checkmate 648
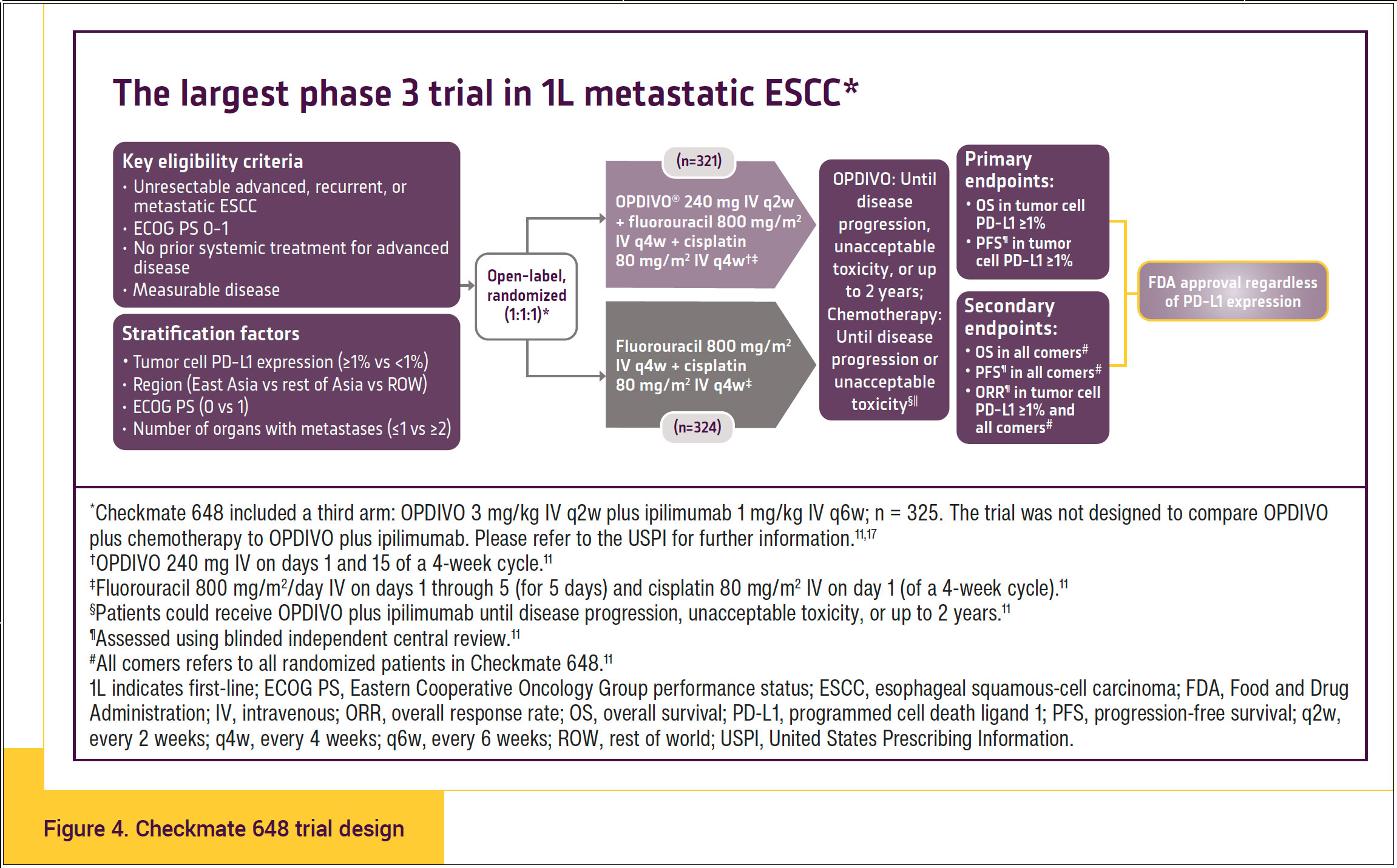
Checkmate 648 was the largest phase 3 trial in first-line metastatic ESCC. Adults with previously untreated, unresectable advanced, recurrent, or metastatic ESCC were enrolled regardless of tumor cell PD-L1 expression. Patients were randomized to OPDIVO (240 mg every 2 weeks) plus chemotherapy (fluorouracil plus cisplatin every 4 weeks) or chemotherapy alone. Patients were also randomized to an experimental arm studying OPDIVO (3 mg/kg every 2 weeks) plus YERVOY® (ipilimumab) (1 mg/kg every 6 weeks). The primary end points for both comparisons were OS and PFS as defined by blinded independent central review in patients with tumor cell PD-L1 ≥1%. Hierarchically tested secondary end points included OS and PFS in all randomized patients (Figure 4).11,17,18
- Patients were also randomized to an experimental arm studying OPDIVO 3 mg/kg every 2 weeks in combination with ipilimumab 1 mg/kg every 6 weeks.11
- In patients who received OPDIVO in combination with chemotherapy and in whom either fluorouracil and/or cisplatin was discontinued, other components of the treatment regimen were allowed to be continued.11 Patients who discontinued combination therapy because of an adverse reaction attributed to ipilimumab were permitted to continue OPDIVO as a single agent.
- Tumor cell score, also called PD-L1 tumor proportion score (TPS), was evaluated using the PD-L1 IHC 28-8 pharmDx assay.11
- The efficacy analysis in patients with PD-L1 TPS ≥1% included 158 patients in the OPDIVO plus chemotherapy arm, 158 patients in the OPDIVO plus ipilimumab arm, and 157 patients in the chemotherapy arm.11
- The trial excluded patients with brain metastasis who were symptomatic, had active autoimmune disease, used systemic corticosteroids or immunosuppressants, or were at high risk of bleeding or fistula due to apparent invasion of tumor into organs adjacent to the esophageal tumor.11
Approximately 49% of patients had tumor cell PD-L1 expression ≥1%, and 51% had PD-L1 expression <1%. Patients with metastatic disease comprised 57% to 58% of the patients in the study.
At a prespecified primary analysis with a minimum follow-up of 12 months, OPDIVO plus chemotherapy resulted in significant improvements in OS and PFS compared with chemotherapy alone in all comers, including PD-L1 expressors and non-expressors.
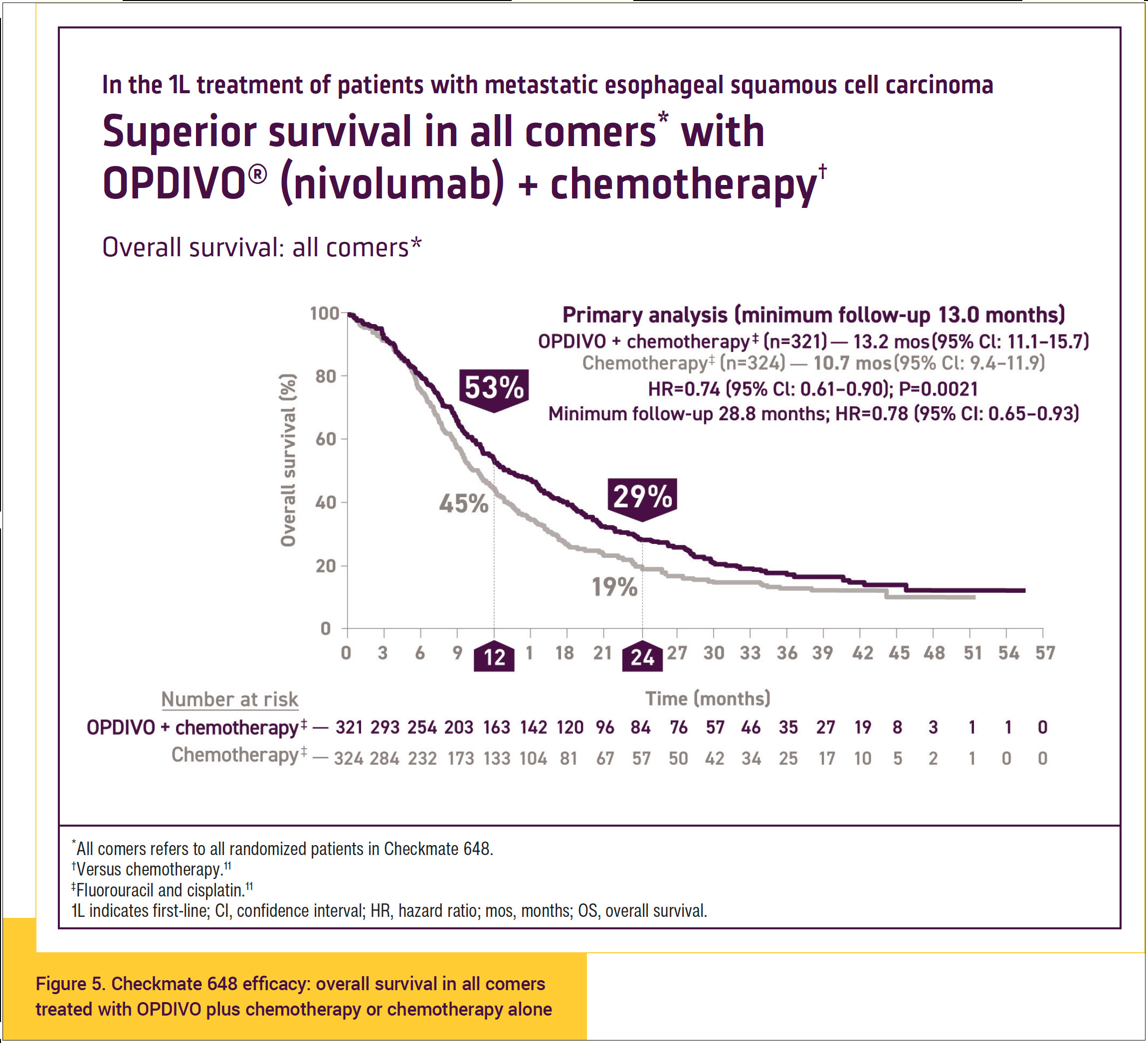
At 12 months, OS was 53% in patients who were treated with OPDIVO plus chemotherapy and 45% in those who were treated with chemotherapy alone (Figure 5).11,17
Dual primary end points in the PD-L1 TPS ≥1% population (N = 315) showed the following:
- Median OS was 15.4 months (95% CI, 11.0-19.5) with OPDIVO plus chemotherapy and 9.1 months (95% CI, 7.7-10.0) with chemotherapy alone (HR, 0.54; 95% CI, 0.41-0.71; P <.0001).
- Median PFS was 6.9 months (95% CI, 5.7-8.3) with OPDIVO plus chemotherapy and 4.4 months (95% CI, 2.9-5.9) with chemotherapy alone (HR, 0.65; 95% CI, 0.49-0.86; P = .0023).
A secondary end point in the study was median PFS in all comers (N = 645), which was 5.8 months (95% CI, 5.6-7.0) with OPDIVO plus chemotherapy and 5.6 months (95% CI, 4.3-5.9) with chemotherapy alone (HR, 0.81; 95% CI, 0.67-0.99; P = not significant).
Overall, these data indicate that there was a 26% reduction in the risk of death with OPDIVO plus chemotherapy versus chemotherapy alone in the all-comers population.
In the analysis at 12 months in Checkmate 648, complete response rates were numerically higher in all comers who were treated with OPDIVO plus chemotherapy compared with chemotherapy alone.
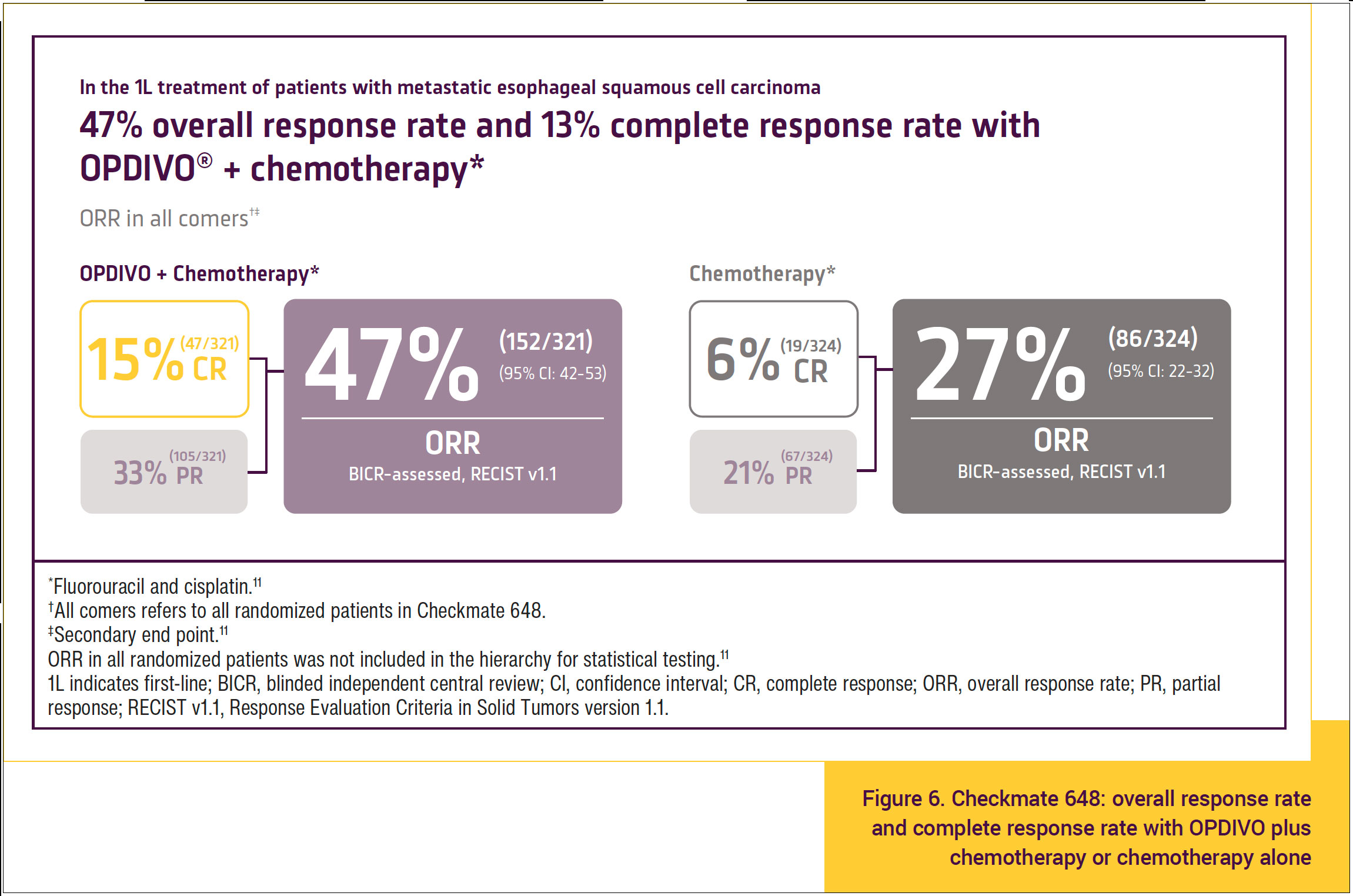
In all comers, there was a 15% complete response rate and a 47% overall response rate in the group treated with OPDIVO plus chemotherapy compared with a 6% complete response rate and a 27% overall response rate in the group treated with chemotherapy alone (Figure 6).11 This suggests a benefit of OPDIVO plus chemotherapy compared with chemotherapy alone in all comers with respect to achieving a complete response.
Moreover, the duration of response in all comers was 8.2 months (95% CI, 6.9-9.7) with OPDIVO plus chemotherapy and 7.1 months (95% CI, 5.7-8.2) with chemotherapy alone.
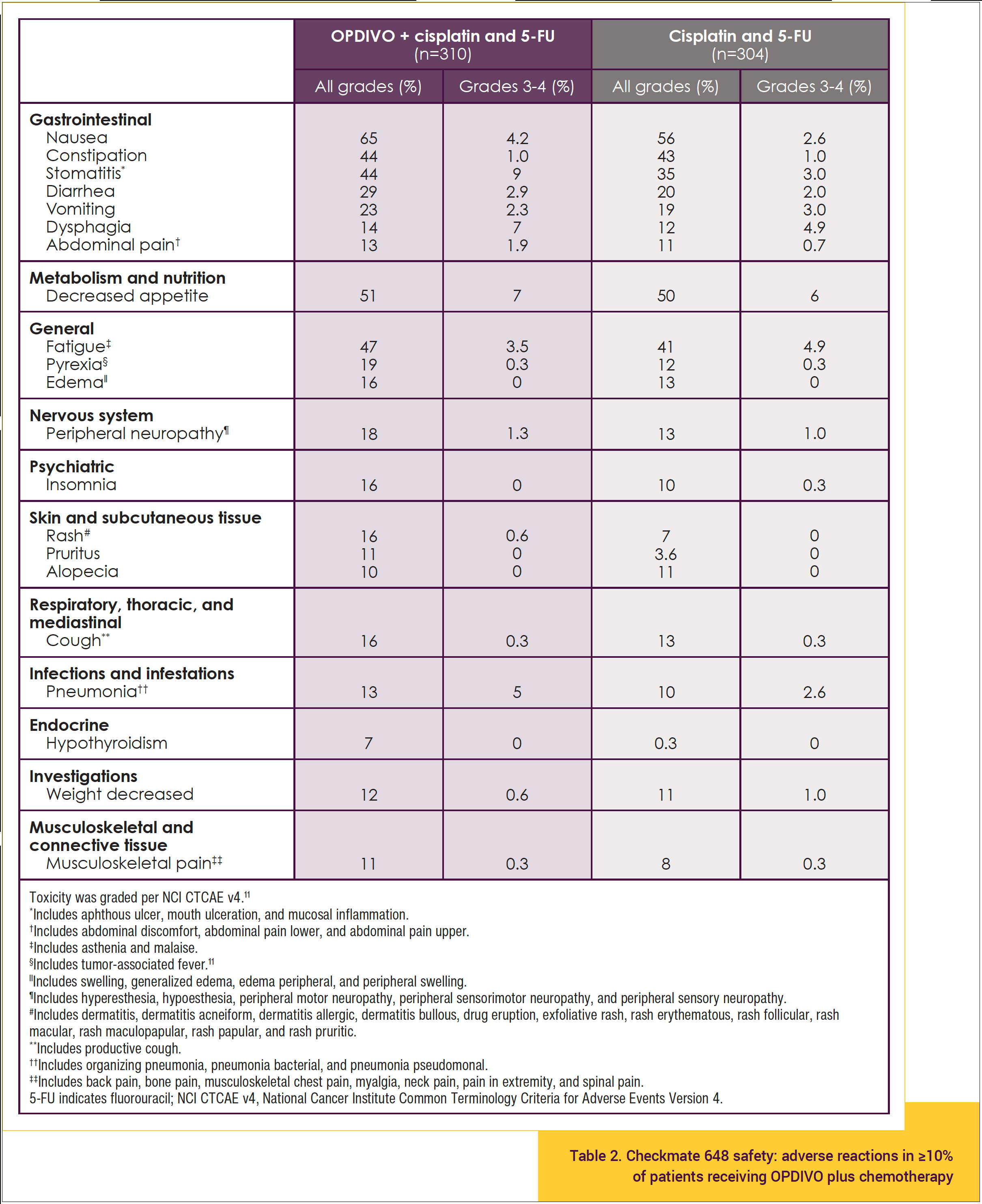
The safety profile of OPDIVO plus chemotherapy shown in Table 211,17 is consistent with the known safety profiles of the individual treatments, and no new safety signals were identified. In Checkmate 648, among patients who received OPDIVO plus chemotherapy:
- Fatal adverse reactions occurred in 5 (1.6%) patients who received OPDIVO in combination with chemotherapy; these included pneumonitis, pneumonitis intestinal, pneumonia, and acute kidney injury.11
- Serious adverse reactions occurred in 62% of patients receiving OPDIVO in combination with chemotherapy.11
- OPDIVO and/or chemotherapy was discontinued in 39% of patients and delayed in 71% of patients because of an adverse reaction.11
- The most frequent serious adverse reactions reported in ≥2% of patients who received OPDIVO with chemotherapy were pneumonia (11%), dysphagia (7%), esophageal stenosis (2.9%), acute kidney injury (2.9%), and pyrexia (2.3%).11
- The most common adverse reactions reported in ≥20% of patients treated with OPDIVO in combination with chemotherapy were nausea (65%), decreased appetite (51%), fatigue (47%), constipation (44%), stomatitis (44%), diarrhea (29%), and vomiting (23%).11
Chemoimmunotherapy is becoming a new standard-of-care treatment for many advanced cancers, and now with the data from the Checkmate 649 and Checkmate 648 studies, OPDIVO plus chemotherapy can be used safely and effectively as first-line therapy in all comers with advanced or metastatic gastric adenocarcinoma, gastroesophageal adenocarcinoma, and esophageal adenocarcinoma as well as those with advanced, unresectable esophageal squamous-cell carcinoma, regardless of PD-L1 status.
References
- American Cancer Society. Key statistics for esophageal cancer. 2022. www.cancer.org/cancer/esophagus-cancer/about/ key-statistics.html. Accessed July 25, 2022.
- American Cancer Society. Key statistics about stomach cancer. 2022. www.cancer.org/cancer/stomach-cancer/about/key-statistics.html. Accessed November 15, 2022.
- Zhou J-H, Li C-Y, Bao J, Zheng G-Q. High expression of long noncoding RNA Sox2ot is associated with the aggressive progression and poor outcome of gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20:4482-4486.
- Decision Resources Group: Gastric Cancer Epidemiology – United States. Princeton, NJ: Bristol-Myers Squibb Company; 2020.
- Shankaran V, Xiao H, Bertwistle D, et al. A comparison of real-world treatment patterns and clinical outcomes in patients receiving first-line therapy for unresectable advanced gastric or gastroesophageal junction cancer versus esophageal adenocarcinomas. Adv Ther. 2021;38:707-720.
- US Food and Drug Administration. FDA approves nivolumab in combination with chemotherapy for metastatic gastric cancer and esophageal adenocarcinoma. 2021. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-combination-chemotherapy-metastatic-gastric-cancer-and-esophageal. Accessed March 15, 2022.
- Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous cell carcinoma. N Engl J Med. 2022;386:449-462.
- Chau J, Ajani JA, Doki Y, et al. Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): Expanded efficacy and safety analyses from CheckMate 648. J Clin Oncol. 2022;40(suppl 16):4035.
- ClinicalTrials.gov. Efficacy study of nivolumab plus ipilimumab or nivolumab plus chemotherapy against chemotherapy in stomach cancer or stomach/esophagus junction cancer (CheckMate649). Updated June 1, 2021. https://clinicaltrials.gov/ct2/show/NCT02872116. Accessed March 15, 2022.
- Janjigian YY, Shitara K, Koehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40.
- OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2023.
- Janjigian YY, Shitara K, Moehler MM, et al. Nivolumab (NIVO) plus chemotherapy (chemo) as first-line (1L) treatment for advanced gastric cancer /gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): 3-year follow-up from CheckMate 649. J Clin Oncol. 2023;41(suppl 4):291.
- Yanjigian YY, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: Checkmate 649 study. Presented at: 2021 European Society for Medical Oncology Congress; September 16-21, 2021. Presentation No. LBA7.
- Data on file. BMS-REF-NIVO-0120. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
- Data on file. BMS-REF-NIVO-0097. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
- Data on file. BMS-REF-ONCO-0014. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
- Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449-462.
- Chau I, Doki Y, Ajani JA, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. Presented at: ASCO 2022. Abstract LBA4001.
- Siewert JR, Stein HJ. Carcinoma of the cardia: carcinoma of the gastroesophageal junction—classification, pathology, and extent of resection. Dis Esophagus. 1996;9:173-182.