The Third Annual World Cutaneous Malignancies Congress (WCMC) will take place in San Francisco, California, on October 29-31, 2014. The WCMC is a 2-day congress dedicated to informing, educating, and fostering the exchange of clinically relevant information in the field of cutaneous malignancies on topics in melanoma, basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, and cutaneous T-cell lymphoma. The following abstracts will be presented at the meeting.
CATEGORY I: Epidemiology and risk factors
A Study of Basal Cell Carcinoma in South Asians for Risk Factor and Clinicopathological Characterization – A Hospital-Based Study
Sumir Kumar,1 B. B. Mahajan,1 Sandeep Kaur,1 Amarbir Singh1
1GGS Medical College & Hospital, Faridkot, Punjab, India
Objectives: Basal cell carcinoma (BCC) is the most common cutaneous malignancy worldwide. Methods: A hospital-based study was conducted in Punjab, North India, from 2011-2013. All patients visiting skin department with suspected lesions underwent history, examination and histopathological confirmation. The results were analyzed using appropriate statistical tests. Results: Total of 36 confirmed cases were seen from 2011-2013. The disease had predilection for females (63.9%) and elderly (47.2% of cases aged 61-80 years) with mean ± SD age being 60.9 ± 14.2 years (65.92 ± 14.35 years for males and 57.96 ± 13.54 years for females). Mean duration of disease was 4.7 years (range being 5 months to 15 years). Majority of patients were rural (69.4%) and illiterate (80.6%). Though there was statistically significantly higher sun exposure in males compared to females (P value being .000), BCC was more common in females, explainable by intermittent sun exposure (during household work in the open kitchens) in women. There was also seen a statistically significant association between long duration of disease and illiteracy (?2 value = 6.95 and P value = .01) and also, duration with size of lesion (evaluated using ?2 test with P value equal to .004). Majority of patients (88.9%) had a single lesion. Head and neck region was involved in 97.2% of cases, nose being the most common site (50%), with nodular/noduloulcerative morphology in 77.8% of cases. Pigmentation was evident in 22.2% of cases clinically. Nodular variety was the most common histopathological variant (77.8%). Conclusion: This study highlights a paradoxically increasing trend of BCC with female preponderance, preferential involvement of nose and higher percentage of pigmentation in Indians. It is more common in rural and agriculture-based populations. Major risk factors include intermittent rather than constant UV exposure, cultural and lifestyle changes, cosmetic indifference, arsenic and pesticides, improved clinical and diagnostic skills. The increasing cancer burden calls for the need of national screening program. The data collected in this study would serve as a reference for future research and development of preventive strategies.
CATEGORY II: Molecular biology and pathogenesis – implications for therapy
Rituximab-Induced Oral Squamous Cell Carcinoma in a Patient With Pemphigus Vulgaris: Consequence or Coincidence?
Sumir Kumar,1 Nidhi Kamra,1 Amarbir Singh,1 B. B. Mahajan1
1GGS Medical College & Hospital, Faridkot, Punjab, India
Objectives: 1. To assess the association between use of intra-lesional rituximab and development of oral squamous cell carcinoma. 2. To determine the risk of development of oral squamous cell carcinoma in patients with pemphigus vulgaris. Methods: Detailed history and clinical examination was done of a 55-year-old man who had presented with a complaint of ulceration over right lateral border of tongue. He was a known case of pemphigus vulgaris diagnosed 4 years back. He was administered intra-lesional injections of rituximab for his recalcitrant oral lesions present over buccal mucosa and base of the tongue 6 months back. But bolt out of blue he developed oral squamous cell carcinoma with complete resolution of other lesions of pemphigus in oral mucosa. On examination, a single ulcero-indurative lesion was present on the right lateral border of tongue. Routine as well as specific investigations including histopathology and DIF were done. The literature regarding risk of malignancy with rituximab and pemphigus vulgaris was reviewed. Results: Histopathology confirmed the diagnosis of oral squamous cell carcinoma and DIF findings for pemphigus were negative. As per the literature reviewed, most common adverse effect reported with rituximab was infusion-related reactions and those related to immunosuppression. Only few studies evaluated the risk of malignancies with rituximab. The time interval between development of malignancy with rituximab was quoted as 5 months in one of the articles with cutaneous squamous cell carcinoma being the most common malignancy. Role of various desmoglein molecules has been proposed in oral squamous cell carcinoma, and decrease in levels of desmoglein-3 molecule has been suggested to be associated with development of poorly differentiated carcinoma. Conclusion: Though development of SCC might have been the consequence of use of intra-lesional rituximab, with no randomized trials establishing risk of malignancies in pemphigus, it might have been just a mere coincidence. Thus this case serves as a lightning bolt to constitute randomized controlled trials to constitute a good quality evidence for the safe and cautious use of rituximab in pemphigus.
CATEGORY III: Current treatment guidelines and challenges
Primary Vulvovaginal Melanoma. A Twelve-Year Multidisciplinary Team Experience
G. Moreno,1 J. Hurren,2 D. McCormick,3 B. Hughes1
1Dermatology Department, St Mary’s Hospital, Portsmouth, UK; 2Plastics/Surgical Oncology Department, Queen Alexandra Hospital, Portsmouth, UK; 3Histopathology Department, Queen Alexandra Hospital, Portsmouth, UK
Introduction: Vulvovaginal melanoma is a rare skin malignancy with poor prognosis. Early diagnosis and surgical treatment provide the best chance of cure. Close monitoring is essential to recognise early possible recurrences. Objective: We aim to present our experience with diagnosis, treatment and outcome with an emphasis on close monitoring aided by serial medical photography. Methods: A retrospective review of medical and pathology records was conducted and data extracted for analysis. Demographics, clinicopathological and treatment data with overall survival were recorded. Clinical photography (when available) was reviewed. The revised 2002 AJCC classification was used to determine the stage of disease. Results: Between 2002 and 2014, eleven cases of primary vulvovaginal melanoma were identified. Mean age at diagnosis was 65.8 years (range 41-88). Mean time from onset of symptoms to presentation was 6.5 months (1.5-12). Signs and symptoms included dyspigmentation, itch, pain, discharge, ulceration, and bleeding. Medical photography was obtained in 5/11 patients. A mean Breslow depth of 3.2 mm was noted in invasive tumours. Wide local excision was the preferred treatment, while radical surgery was performed in 4/11 to control locally advanced disease. Sentinel lymph node biopsy was performed in 1 patient. Radiation and chemotherapy were largely reserved for palliation of symptoms. Conclusion: Early detection and close monitoring of premalignant lesions can be assisted by serial photography. We have set up this as the standard of care for any vulval and low vaginal melanocytic lesion.
Preliminary Results of a Proof-of-Concept Trial of Intratumoral (IT) Administration of Glucopyranosyl Lipid Adjuvant (GLA), a Toll-Like Receptor-4 (TLR-4) Agonist, in Patients With Merkel Cell Carcinoma (MCC)
Shailender Bhatia,1 Dafina Ibrani,1 Olga Afanasiev,1 Natalie Vandeven,1 David Byrd,1 Upendra Parvathaneni,1 Michael Donahue,1 Frank J. Hsu,2 Barry Storer,1 David Koelle,1 Paul Nghiem1
1University of Washington/Fred Hutchinson Cancer Research Center, Seattle, WA; 2Immune Design, Seattle, WA
Background: MCC is an aggressive skin cancer with limited therapeutic options. Despite persistent expression of the non-self Merkel cell polyomavirus (MCPyV) proteins, MCC tumors are able to evade the immune surveillance mechanisms through multiple interrelated mechanisms: down-regulation of MHC class I expression, strikingly sparse IT infiltrates of T cells, and immune exhaustion of infiltrating T-lymphocytes (TIL). Therapeutic immune modulation of the MCC tumor microenvironment using IT injections of GLA, a synthetic TLR-4 agonist, may overcome these evasion mechanisms via immune responses against the tumor antigens. Objectives: This proof-of-concept trial (NCT02035657) is a single center study to test the safety, clinical efficacy and immunologic effects of IT administration of GLA in MCC patients (pts). Methods: 10 MCC pts will be enrolled to receive multiple IT injections into a superficial injectable tumor. Pts with localized MCC (cohort A) may receive 1 cycle of GLA injections (on days 1, 8) followed by definitive surgery and/or radiation therapy (RT) starting during week 4; pts with distant metastatic disease (cohort B) may receive multiple cycles of GLA injections (days 1, 8, 22) every 6 weeks (up to 4 cycles, may be in conjunction with RT). Serial tumor biopsies and peripheral blood samples will be collected in all pts at baseline and posttreatment. Results: Four pts have been enrolled to date (2 in each cohort). Treatment has been tolerated well with no grade 3/4 or serious adverse events (AEs). Treatment-related AEs have all been grade 1 (inflammation, pain and bruising at the injection site), except transient grade 2 inflammation at the injected inguinal lymph node in patient 002 (in cohort A), who interestingly also had a pathologic complete remission of the involved node after only 2 GLA injections. Both pts in cohort A successfully completed definitive therapy without any delays. Both pts in cohort B successfully completed cycle 1 but had progressive disease at the first restaging evaluation. We identified MCPyV-specific CD8 T cells isolated from TIL and peripheral blood, and correlative immune studies tracking these cells in terms of frequency and functional status are in progress. Conclusion: Preliminary results indicate that IT immunotherapy with GLA injections in MCC patients is tolerated well and may induce antitumor responses. Updated clinical and correlative results will be presented at the meeting.
CATEGORY V: Ongoing pivotal clinical trials
A Phase 2, Randomized, Double-Blind Study of Sonidegib (LDE225) in Patients (pts) With Advanced Basal Cell Carcinoma (BCC): BOLT 12-Month Update
Michael Migden,1 Alexander Guminski,2 Ralf Gutzmer,3 Luc Dirix,4 Karl Lewis,5 Patrick Combemale,6 Robert Herd,7 Sven Gogov,8 Tingting Yi,9 Manisha Mone,9 Ragini Kudchadkar,10 Uwe Trefzer,11 John Lear,12 Dalila
Sellami,9 Reinhard Dummer13
1The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 2Royal North Shore Hospital, St Leonards, New South Wales, Australia; 3Medizinische Hochschule Hannover, Hannover, Germany; 4Sint-Augustinus Ziekenhuis, Antwerp, Belgium; 5University of Colorado Cancer Center, Aurora, CO, USA; 6Centre Leon Bérard, Lyon, France; 7Glasgow Royal Infirmary, Glasgow, UK; 8Novartis Pharma AG, Basel, Switzerland; 9Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 10Moffitt Cancer Center, Tampa, FL, USA; 11Dermatologikum Berlin, Berlin, Germany; 12Manchester Academic Health Science Centre, University of Manchester, Manchester, UK; 13UniversitätsSpital Zürich – Skin Cancer Center University Hospital, Zürich, Switzerland
Hedgehog (Hh) signaling, aberrantly activated in ?95% of BCCs, is blocked by sonidegib, a selective smoothened inhibitor. BOLT, a study of 2 dosages of sonidegib in pts with advanced BCC (NCT01327053), met its primary end point of objective response rate (ORR) ?30% (locally advanced BCC [LaBCC] and metastatic BCC [mBCC] combined) in both arms using data collected up to 6 mo after the last pt randomization (cutoff, Jun 28, 2013; median follow-up, 13.9 mo; Migden, ASCO 2014). Pts with LaBCC (n=194), not amenable to curative surgery or radiation, or mBCC (n=36) were randomized 1:2 to receive sonidegib 200 or 800 mg daily. ORR (complete response [CR] or partial response [PR]), CR rate, time to tumor response (TTR), duration of response (DOR), and progression-free survival (PFS) were assessed according to modified RECIST (LaBCC) or RECIST v1.1 (mBCC). Efficacy and safety data up to 12 mo after the last pt randomization (cutoff, Dec 31, 2013; median follow-up, 20.0 mo) are reported. Median duration of exposure was 11.0 (200 mg) and 6.6 (800 mg) mo. ORRs (95% CI) per central review for 200 vs 800 mg were 57.6% (44.8%-69.7%; n=66) vs 43.8% (35.0%-52.8%; n=128) for LaBCC and 7.7% (0.2%-36.0%; n=13) vs 17.4% (5.0%-38.8%; n=23) for mBCC. Respective ORRs (95% CI) per investigator review were 71.2% (58.7%-81.7%; n=66) vs 57.8% (48.8%-66.5%; n=128) and 23.1% (5.0%-53.8%; n=13) vs 34.8% (16.4%-57.3%; n=23). Remaining data are per investigator. Disease control (CR + PR + stable disease) rates for 200 vs 800 mg were 92.4% vs 85.9% (LaBCC) and 84.6% vs 82.6% (mBCC). Median TTR (95% CI) for 200 vs 800 mg was 2.5 (1.9-3.7) vs 1.9 (1.4-2.0) mo for LaBCC and 1.0 (0.9-3.7) vs 2.7 (1.0-5.6) mo for mBCC. Median DOR (95% CI) was 20.2 (not estimable [NE]; 200 mg) and 19.8 (15.7-20.5; 800 mg) mo for LaBCC and 17.7 (NE; 200 mg) and 10.2 (NE; 800 mg) mo for mBCC. Median PFS (95% CI) was 22.0 (NE; 200 mg) and 21.5 (NE; 800 mg) mo for LaBCC and 13.1 (9.2-18.6; 200 mg) and 14.3 (11.1-20.2; 800 mg) mo for mBCC. The safety findings remained consistent with longer follow-up. Adverse events (AEs) were manageable; the most common AEs (any grade; 200/800 mg) included muscle spasms (52%/69%), alopecia (49%/57%), dysgeusia (41%/60%), and nausea (35%/47%). An additional 6 mo of follow-up provides further evidence that sonidegib treatment leads to meaningful disease control, with sustained DOR and prolonged PFS in pts with advanced BCC. The 200-mg dose had a more favorable benefit-risk profile.
BRIM8: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Vemurafenib Adjuvant Therapy in Patients With Surgically Resected, Cutaneous BRAF-Mutant Melanoma at High Risk for Recurrence (NCT01667419)
Grant R. Goodman,1 Dirk Schadendorf,2 Michele Maio,3 Mario Mandalà,4 Betty J. Nelson,1 Karl D Lewis5
1Genentech, Inc, South San Francisco, California, USA; 2University Hospital Essen, Essen, Germany; 3Papa Giovanni XXIII Hospital, Bergamo, Italy; 4University Hospital, Siena, Italy; 5University of Colorado, Denver, Aurora, Colorado, USA
Approximately 50% of melanomas have a mutation in the BRAF gene. The oral BRAF inhibitor vemurafenib has shown meaningful clinical benefit in BRAF V600–mutated, locally advanced/unresectable or metastatic melanoma. For patients with resected melanoma, interferon ?-2b is the only widely approved adjuvant therapy; however, its use is limited by modest improvement in disease recurrence and high incidence of severe adverse effects that lead to treatment discontinuation in up to one-third of patients. BRIM8 is a phase 3, international, multicenter, double-blind, randomized, placebo-controlled study to evaluate the safety and efficacy of adjuvant vemurafenib therapy. Patients ?18 years with completely resected, histologically confirmed, stage IIC or III BRAF V600 mutation–positive (as detected by the cobas® BRAF V600 Mutation Test [Roche Molecular Diagnostics]) melanoma of cutaneous origin are eligible. Patients without evidence of regional lymph node involvement must undergo sentinel lymph node biopsy, and those with evidence of regional or sentinel lymph node involvement must undergo complete regional lymphadenectomy. Patients with a history of systemic therapy for treatment or prevention of melanoma are ineligible. Two cohorts (C) will enroll 725 patients: C1, 500 patients with stage IIC, IIIA (nodal metastasis >1 mm in diameter), or IIIB cutaneous melanoma; C2, 225 patients with stage IIIC cutaneous melanoma. Patients will be randomized 1:1 to receive vemurafenib (960 mg bid) or placebo for 52 weeks, with randomization stratified by stage and region (C1) or by region alone (C2). Primary efficacy outcome measure is investigator-assessed disease-free survival. Secondary efficacy outcome measures include overall and distant metastasis-free survival. Safety, pharmacokinetic, and patient-reported outcomes will also be assessed.
Reused with permission from the American Society of Clinical Oncology (ASCO). This abstract was accepted and previously presented at the 2014 ASCO Annual Meeting.
NEMO: A Phase 3 Trial of Binimetinib (MEK162) Versus Dacarbazine in Patients With Advanced NRAS-Mutant Melanoma Who Are Untreated or Have Progressed After Any Number of Immunotherapy Regimens
Reinhard Dummer,1 Paolo A. Ascierto,2 Georgina V. Long,3 Dirk Schadendorf,4 Ana Arance,5 Petr Arenberger,6 Lev Demidov,7 Ralf Gutzmer,8 Mario Mandalà,9 Michele Maio,10 Frank Meiss,11 Piotr Rutkowski,12 Pascal Wolter,13 Naoya Yamazaki,14 Ernesto Wasserman,15 James Ford,15 Marine Weill,15 Keith Flaherty16
1University Hospital Zurich, Zurich, Switzerland; 2Istituto Nazionale Tumori Fondazione Pascale, Naples, Italy; 3Melanoma Institute Australia and The University of Sydney, Sydney, Australia; 4University Hospital Essen, Essen, Germany; 5Hospital Clínic, Barcelona, Spain; 6Charles University, 3rd Medical Faculty, Prague, Czech Republic; 7N. N. Blokhin Russian Cancer Research Center, Moscow, Russia; 8Hannover Skin Cancer Center, Hannover Medical School, Hannover, Germany; 9Papa Giovanni XXIII Hospital, Bergamo, Italy; 10University Hospital of Siena, Siena, Italy; 11Freiburg University Medical Center, Freiburg, Germany; 12Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland; 13University Hospitals Leuven, Leuven Cancer Institute, Leuven, Belgium; 14National Cancer Center Hospital, Tokyo, Japan; 15Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 16Massachusetts General Hospital, Boston, MA, USA.
Background: The tumors of patients with melanoma often harbor mutations in the mitogen-activated protein kinase-signaling pathway family members BRAF or NRAS. Mutations in the NRAS gene are observed in about 20% of melanoma cases. Compared with BRAF-mutant and wild-type disease, NRAS-mutant melanoma has been associated with increased proliferation, thicker primary tumors, higher rates of brain metastasis, and poorer prognosis. In a large retrospective analysis (n=677), NRAS mutations were shown to be independently predictive of poor survival in patients with cutaneous melanoma (Jakob et al, 2011). There are no approved targeted therapies specifically for NRAS-mutant melanoma, and treatments are currently limited to chemotherapy and/or immunotherapy in this patient subset. Binimetinib (MEK162) is a potent and selective inhibitor of MEK1/2 that has demonstrated promising phase 2 clinical activity in NRAS-mutant melanoma. Here we describe the “NRAS mElanoma and MEK inhibitOr” (NEMO) trial, an ongoing phase 3 study designed to compare the efficacy of binimetinib vs dacarbazine in patients with metastatic NRAS-mutant melanoma (NCT01763164). Trial Design: The NEMO trial is a 2-arm, open-label, 2:1 randomized trial comparing binimetinib vs dacarbazine in patients with metastatic NRAS-mutant melanoma. Eligible patients must have advanced unresectable or metastatic cutaneous melanoma or melanoma of unknown primary origin with a documented NRAS Q61 mutation (by central molecular screening) that was previously untreated or has progressed after any number of immunotherapy regimens. Patients are stratified by stage, performance status, and prior immunotherapy. The primary end point of the study is progression-free survival, and secondary end points include overall survival, overall response, disease control rate, safety, and quality of life. Patients receive oral binimetinib at 45 mg twice daily or dacarbazine dosed intravenously at 1000 mg/m2 once every 3 weeks. The phase 3 NEMO trial is designed to enroll 393 patients and is currently recruiting patients at more than 150 centers worldwide. The overall study design, eligibility criteria, methodology, and end points of this trial will be presented.
CATEGORY VI: Targeted therapy
Safety and Efficacy of Vismodegib in Patients With Basal Cell Carcinoma Nevus Syndrome: Analysis of the ERIVANCE BCC and Expanded Access Studies
Anne L.S. Chang,1 Sarah T. Arron,2 Michael R. Migden,3 James A. Solomon,4-6 Simon Yoo,7 Bann-Mo Day,8 Edward McKenna,8 Aleksandar Sekulic9
1Stanford University, Redwood City, California, USA; 2University of California, San Francisco, San Francisco, California, USA; 3The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 4Ameriderm Research, Ormond Beach, Florida, USA; 5University of Central Florida, Orlando, Florida, USA; 6University of Illinois, Urbana, Illinois, USA; 7Northwestern University, Chicago, Illinois, USA; 8Genentech, Inc, South San Francisco, California, USA; 9Mayo Clinic, Scottsdale, Arizona, USA
Background: Aberrant activation of the Hedgehog pathway (HhP) is a key driver in the pathogenesis of basal cell carcinoma (BCC), including patients (pts) with BCC nevus syndrome (BCCNS). Vismodegib (VISMO), the first HhP inhibitor to be FDA approved, represents a new treatment option for pts with advanced (a) BCC, including local advanced (laBCC) or metastatic (mBCC) disease. VISMO is indicated for pts with aBCC that has recurred after surgery or who are not candidates for surgery and radiation. We present the efficacy and safety of VISMO in BCCNS pts with aBCC across 2 trials – ERIVANCE BCC trial and the expanded access study (EAS). Methods: ERIVANCE BCC and EAS enrolled pts with aBCC. The EAS was terminated early upon FDA approval of VISMO. All pts received oral VISMO 150 mg daily until disease progression or intolerable toxicity. Response in both studies for mBCC pts was evaluated using RECIST v1.0. Response for laBCC pts was evaluated by a novel composite end point in ERIVANCE BCC and by RECIST v1.0 in the EAS. Response was assessed every 8 wks in ERIVANCE BCC and every 8-16 wks in the EAS. Safety assessments (NCI-CTCAE v3.0) were performed monthly in both trials. BCCNS diagnosis was based on medical history at enrollment. Due to described differences in response assessment/schedule, pts with BCCNS were not pooled across trials. Analytic cohorts for BCCNS and sporadic aBCC were created within each trial for comparison using descriptive statistical methods. Results: 22/0 and 12/7 BCCNS pts (laBCC/mBCC) were enrolled in ERIVANCE BCC and EAS, respectively. Baseline characteristics included median age (47/52 years), female sex (45%/50%), ECOG PS 0-1 (100%/100%), prior surgery (96%/100%), prior radiotherapy (5%/8%), and prior systemic therapy (23%/17%). Median treatment durations were 10.5 and 5 months, respectively, and similar to treatment duration of sporadic aBCC pts within each trial. Best objective response rate (BORR) (investigator assessed) in laBCC cohorts was 81% (17/21 pts) in ERIVANCE BCC and 33% (4/12 pts) in EAS. The BORR for the sporadic laBCC cohort was 50% in both studies. No BCCNS pts with mBCC were enrolled in ERIVANCE BCC. BORR in the EAS mBCC cohort was 50% (3/6 pts) compared with 46% and 27% in sporadic mBCC cohorts in ERIVANCE BCC and EAS, respectively. Most frequent adverse events (AEs) included alopecia (86%/58%), muscle spasms (77%/63%), weight decrease (68%/5%), and dysgeusia (59%/74%). 9 (41%) and 2 (16%) pts had grade ?3 AEs in each study, respectively. Incidence and severity of AEs were comparable with the sporadic aBCC cohort of both trials. Conclusion: VISMO demonstrated clinical activity across analytic cohorts and studies. Observed variability (ORR and AEs) across cohorts is likely due to small sample size, differences in response criteria, assessment schedule, treatment duration, and length of follow-up. The safety profile of VISMO was similar between pts with BCCNS and pts with sporadic aBCC.
Safety and Efficacy of Vismodegib in Elderly Patients: Analysis of the ERIVANCE BCC and
Expanded Access Studies
Anne L.S. Chang,1 Karl Lewis,2 Sarah T. Arron,3 Michael R. Migden,4 James A. Solomon,5-7 Simon Yoo,8 Bann-Mo Day,9 Edward McKenna,9 Aleksandar Sekulic10
1Stanford University, Redwood City, California, USA; 2University of Colorado Cancer Center, Aurora, Colorado, USA; 3University of California, San Francisco, San Francisco, California, USA; 4The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 5Ameriderm Research, Ormond Beach, Florida, USA; 6University of Central Florida, Orlando, Florida, USA; 7University of Illinois, Urbana, Illinois, USA; 8Northwestern University, Chicago, Illinois, USA; 9Genentech, Inc, South San Francisco, California, USA; 10Mayo Clinic, Scottsdale, Arizona, USA
Methods: ERIVANCE BCC and EAS enrolled pts with laBCC or mBCC. The EAS was terminated early upon FDA approval of VISMO. All pts received oral VISMO 150 mg daily until disease progression/intolerable toxicity. Safety assessments (NCI-CTCAE v3.0) were performed monthly in both trials. Efficacy assessment was (1) RECIST v1.0 for mBCC in both studies, (2) novel composite end point for laBCC in ERIVANCE BCC and by RECIST v1.0 for laBCC in EAS. Response assessments were performed every 8 weeks in ERIVANCE BCC and every 8-16 weeks in EAS. Because of differences in treatment duration and response assessment, data were not pooled across trials. Analytic cohorts for pts aged <65 and ?65 years were created within each trial for comparison using descriptive statistical methods. Background: Aberrant activation of the Hedgehog pathway (HhP) is a key driver in the pathogenesis of basal cell carcinoma (BCC). Vismodegib (VISMO), the first HhP inhibitor to be FDA approved, is a new treatment option for patients (pts) with advanced (a) BCC and is indicated for pts with aBCC (including locally advanced [la] or metastatic [m] disease) that has recurred after surgery or who are not candidates for surgery and radiation. BCC, particularly aBCC, are prevalent among the elderly. We present analyses of the safety and efficacy of VISMO in pts aged ?65 years vs pts <65 years across 2 trials – ERIVANCE BCC trial and the expanded access study (EAS). Results: 104 aBCC pts were enrolled in ERIVANCE BCC (33 mBCC/71 laBCC) and 119 aBCC pts were enrolled in the EAS (57 mBCC/62 laBCC). ERIVANCE BCC included 57 pts <65 years and 47 pts ?65 years. The EAS included 66 pts <65 years and 53 pts ?65 years. Of laBCC pts aged <65 vs ?65 years in ERIVANCE BCC, 45% vs 46% were female. In the EAS, 43% vs 15% were female. Of the mBCC pts aged <65 vs ?65 years in ERIVANCE BCC, 26% vs 29% were female. In the EAS, 26% vs 15% were female. Median treatment durations in aBCC pts aged <65 and ?65 years were 10.2 and 9.2 months in ERIVANCE BCC, respectively, and 5.4 and 5.5 months in EAS, respectively. The most frequent adverse events (AEs) in aBCC pts aged <65 vs ?65 years in ERIVANCE BCC and the EAS, respectively, were muscle spasms (72% vs 64%/71% vs 70%), dysgeusia (51% vs 51%/73% vs 70%), and alopecia (75% vs 49%/61% vs 55%). Grade 3-5 AEs in aBCC pts aged <65 vs ?65 years occurred in 35% vs 51% in ERIVANCE BCC and 21% vs 25% in EAS, respectively. Similarly, AEs leading to treatment discontinuation occurred in 11% vs 15% and 2% vs 11% of aBCC pts aged <65 vs ?65 years, respectively. Best objective response rates (BORRs) by investigator (INV) assessment were 73% and 47% in laBCC pts aged <65 years and 47% and 46% in laBCC pts aged ?65 years in ERIVANCE BCC and EAS, respectively. BORRs by INV assessment were 53% and 29% in mBCC pts aged <65 years and 36% and 33% in mBCC pts aged ?65 years in ERIVANCE BCC and EAS, respectively. Conclusion: VISMO demonstrated similar safety profiles and clinical activity in pts <65 and ?65 years across both studies.
Treatment of Basal Cell Carcinoma With Oral Vismodegib Preceding Mohs Micrographic Surgery Excision: Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study (NCT01898598)
Abel Torres,1 Jean Tang,2 Sarah Arron,3 Michael Migden,4 Clay Cockerell,5 Steve Lee,6 Edward McKenna,6 Diana Chen6
1Loma Linda University Medical Center, Loma Linda, California, USA; 2Stanford University Medical Center, Redwood City, California, USA; 3University of California, San Francisco, San Francisco, California, USA; 4University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 5University of Texas Southwestern Medical Center, Dallas, Texas, USA; 6Genentech, Inc, South San Francisco, California, USA
Background: Basal cell carcinoma (BCC) is the most common malignancy in the United States. Almost all sporadic BCCs have mutations in the Hedgehog (Hh) signaling pathway. Vismodegib is an oral Hh pathway inhibitor approved by the FDA for the treatment of adults with metastatic BCC, or with locally advanced BCC that has recurred following surgery, or who are not candidates for surgery or radiation therapy. Treatment goals for operable BCC focus on complete tumor removal and minimization of functional and aesthetic defects. Mohs micrographic surgery (MMS) represents the most definitive treatment method for BCC, with visualization of the entire surgical margin ensuring complete tumor removal with maximal preservation of surrounding tissue. Clinical studies have demonstrated that vismodegib is safe and effective in shrinking the majority of locally advanced BCC tumors; however, it is unknown whether vismodegib may be used as an adjunct to surgery and if discontiguous subclinical tumor (skip areas) remains following treatment. This study will enroll patients whose target BCC may be excised by MMS to evaluate whether vismodegib can safely and effectively be used before surgery to shrink tumors and reduce the surgical defect size. Methods: The trial will consist of a 12-week study drug treatment period followed by MMS within 2 weeks of the last study drug treatment, postsurgical follow-up visits to assess for recurrence, and a 52-week follow-up period for safety after the final dose of study drug. Men and women at least 18 years of age with an untreated, biopsy-confirmed target BCC at least 50 mm2 and less than 2.0 cm in diameter will be enrolled. Patients will be randomly assigned 2:1 to receive once-daily oral vismodegib or placebo and stratified at randomization by presence or absence of morpheaform/infiltrative BCC subtype. Computer-aided planimetry of the entire original tumor area will be used to measure expected and actual surgical defect areas. After the Mohs surgeon has established clear margins, MMS tissue specimens will be submitted to an independent dermatopathologist for determination of skip areas. The primary objective will be to compare the efficacy of vismodegib to placebo as adjunctive presurgical treatment before MMS, as assessed by the percentage change in expected surgical defect area at the MMS excision. Secondary objectives will include an assessment of the actual tumor-free excision area, presence/absence of skip areas, and safety. An interim analysis will be performed when the first 38 patients have completed the MMS visit or withdrawn early. The study is currently open at 19 sites.
Hedgehog Pathway Inhibition With Sonidegib (LDE225) in Patients With Advanced Basal Cell Carcinoma
Reinhard Dummer,1 Ralf Gutzmer,2 Michael Migden,3 Martin Kaatz,4 Carmen Loquai,5 Alex Stratigos,6 Hans-Joachim Schulze,7 Ruth Plummer,8 Celine Pallaud,9 Sven Gogov,9 Mahtab Marker,10 Manisha Mone,10 Anne Lynn S. Chang,11 Frank Cornélis,12 John Lear,13 Dalila Sellami,10 Alexander Guminski14
1UniversitätsSpital Zürich – Skin Cancer Center University Hospital, Zürich, Switzerland; 2Medizinische Hochschule Hannover, Hannover, Germany; 3University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 4SRH Wald-Klinikum Gera GmbH, University Hospital Jena, Gera, Germany; 5University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany; 6Andreas Sygros Hospital, University of Athens, Athens, Greece; 7Fachklinik Hornheide, Münster, Germany; 8Northern Centre for Cancer Care, Freeman Hospital, Newcastle Upon Tyne, UK; 9Novartis Pharma AG, Basel, Switzerland; 10Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA; 11Stanford University School of Medicine, Redwood City, California, USA; 12Cliniques Universitaires Saint-Luc, Bruxelles, Belgium; 13Manchester Academic Health Science Centre, University of Manchester, Manchester, UK; 14Royal North Shore Hospital, St Leonards, New South Wales, Australia
The Hedgehog (Hh) pathway is aberrantly activated in ?95% of basal cell carcinomas (BCCs); expression of GLI1 is a marker for Hh pathway activation. Sonidegib, which blocks Hh signaling by inhibiting smoothened, provided meaningful disease control in patients (pts) with advanced BCC in a phase 2 study (BOLT; NCT01327053). Associations of GLI1 levels with clinical outcomes are presented here. Pts with locally advanced BCC (LaBCC) not amenable to curative surgery or radiotherapy (n=194) or metastatic BCC (mBCC; n=36) were randomized 1:2 to receive sonidegib 200 or 800 mg once daily. Clinical response was assessed by central review using modified RECIST (LaBCC) or RECIST 1.1 (mBCC). Exploratory analyses were performed, including assessment of GLI1 levels in tumor samples (137 LaBCC; 13 mBCC) at baseline (BL), wk 9, and wk 17 by qRT-PCR; tumor presence in samples was confirmed prior to GLI1 analyses. GLI1 levels were reduced from BL at wk 9 and 17 with both sonidegib doses (Table). With adjustments for dose, BCC subtype, and multiple testing, similar GLI1 BL levels and decreases from BL at wk 9 and 17 were seen for LaBCC (aggressive and nonaggressive) and mBCC. At wk 17, reductions from BL in GLI1 levels were seen with both doses in pts with disease control (complete or partial response [CR or PR] or stable disease [SD]). Among pts with greater GLI1 inhibition from BL at wk 17, those in the 800-mg group appeared to have a higher risk of grade ?2 creatine kinase elevation than those in the 200-mg group. Sonidegib substantially reduced GLI1 levels in pts with advanced BCC, with similar results across doses, time points, and BCC subtypes. Pts with disease control showed GLI1 reductions from BL; 1 pt with progressive disease (PD) had increased GLI1 from BL. This supports the potential of sonidegib as an option for treating advanced BCC.

Quality of Life With Sonidegib (LDE225) Treatment in Patients With Advanced Basal Cell Carcinoma
Alexander Guminski,1 Michael Migden,2 Ralf Gutzmer,3 Karl Lewis,4 Luc Dirix,5 Ragini Kudchadkar,6 Robert Herd,7 Sven Gogov,8 Tingting Yi,9 Keiko Higuchi,9 Patrick Combemale,10 Uwe Trefzer,11 John Lear,12 Dalila Sellami,9 Reinhard Dummer13
1Royal North Shore Hospital, St Leonards, New South Wales, Australia; 2The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 3Medizinische Hochschule Hannover, Hannover, Germany; 4University of Colorado Cancer Center, Aurora, Colorado, USA; 5Sint-Augustinus Ziekenhuis, Antwerp, Belgium; 6Moffitt Cancer Center, Tampa, Florida, USA; 7Glasgow Royal Infirmary, Glasgow, United Kingdom; 8Novartis Pharma AG, Basel, Switzerland; 9Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA; 10Centre Leon Bérard, Lyon, France; 11Dermatologikum Berlin, Berlin, Germany; 12Manchester Academic Health Science Centre, University of Manchester, Manchester, UK; 13UniversitätsSpital Zürich – Skin Cancer Center University Hospital, Zürich, Switzerland
Advanced basal cell carcinoma (BCC) can lead to reduced quality of life (QOL) in patients (pts) due to morbidity and disfigurement. The Hedgehog (Hh) pathway is aberrantly activated in ?95% of BCCs; sonidegib blocks the Hh pathway via inhibition of smoothened. In a phase 2 study of sonidegib (BOLT; NCT01327053), meaningful disease control was achieved in pts with advanced BCC. The impact on pt-reported QOL is presented here. Pts with locally advanced BCC (LaBCC) not amenable to curative surgery or radiotherapy (n=194) or metastatic BCC (mBCC; n=36) were randomized in a 1:2 ratio to receive sonidegib 200 or 800 mg daily. Assessment of QOL was based on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (C30) and the associated Head and Neck Cancer module (H&N35). Prespecified subscales included physical functioning, social functioning, pain, and fatigue for C30 and trouble with social contact, head and neck pain, and weight loss for H&N35. C30 and H&N35 were completed at baseline (BL) and ?1 post-BL time by 89% and 90% of pts, respectively. In a majority of pts, scores were maintained or improved compared with BL with both sonidegib doses in LaBCC and mBCC. With 200 mg, improvement in C30 scores was seen in pts (LaBCC, mBCC) for physical functioning (36%, 69%), social functioning (26%, 38%), pain (31%, 46%), and fatigue (38%, 46%); in additional pts, scores were maintained for physical functioning (48%, 23%), social functioning (66%, 46%), pain (59%, 54%), and fatigue (43%, 46%). Improvement in H&N35 scores was seen for trouble with social contact (43%, 31%), head and neck pain (18%, 23%), and weight loss (16%, 17%); scores were maintained in additional pts for trouble with social contact (47%, 54%), head and neck pain (78%, 69%), and weight loss (84%, 67%). In general, maintenance of scores in each scale was observed through week 73 with both doses in a descriptive analysis of mean scores. Median time (mo) to deterioration (>10-point worsening without subsequent improvement) with 200 mg was 13.7 for fatigue, 16.6 for weight loss, and not estimable (NE) for other scales; with 800 mg, median time (mo) to deterioration was 11.1 for physical functioning, 11.3 for social functioning, 5.6 for fatigue, 16.5 for weight loss, and NE for other scales. In summary, pts treated with sonidegib had maintenance or improvement in QOL, supporting the treatment effect observed in the BOLT study and good tolerability of sonidegib.
Longitudinal Safety Modeling and Simulation for Optimization of Vismodegib Treatment Interruption in a Phase 2 Study of Operable Basal Cell Carcinoma
Howard Sofen,1 Tong Lu,2 Ivor Caro,2 Jin Jin2
1UCLA School of Medicine, Los Angeles, California, USA; 2Genentech, Inc, South San Francisco, California, USA
Background: Vismodegib (VISMO), the first FDA-approved Hedgehog pathway inhibitor, is indicated for adults with metastatic or locally advanced basal cell carcinoma (BCC) that has recurred after surgery or who are not candidates for surgery and radiation; it provides a new treatment option for patients (pts) with advanced BCC. Most common adverse drug reactions (ADRs) (>30%) included muscle spasms, alopecia, dysgeusia, weight loss, fatigue, and nausea. To explore the effect of VISMO treatment interruption/discontinuation (I/D) on the ADRs including muscle spasms and dysgeusia/ageusia, we used longitudinally ordered categorical models in a phase 2 study with varying treatment durations and follow-up in pts with operable BCC. Methods: Pts received VISMO (150 mg QD): cohort (C)1, 12 weeks (wks); C2, 12 wks with 24 wks observation (obs); or C3, 8 wks with 4 wks obs then 8 wks. Individual pharmacokinetic (PK) profiles for unbound VISMO were predicted based on a previous population PK model incorporating individual covariates and used in this PK/AE analysis in NONMEM (Laplacian method). Results: 24 pts enrolled in C1, 25 in C2, and 25 in C3. 24 pts in each cohort reported any adverse events, the most frequently reported (>30% in any individual cohort) including: C1, muscle spasms (19 pts; 79%), ageusia (10 pts; 42%), dysgeusia (9 pts; 38%), and alopecia (8 pts; 33%); C2, muscle spasms (19 pts; 76%), alopecia (17 pts; 68%), and dysgeusia (13 pts; 52%); C3, muscle spasms (18 pts; 72%), alopecia (18 pts; 72%), and dysgeusia (15 pts; 60%). The median duration of AEs was approximately 20 days for muscle spasm and 35 days for dysgeusia/ageusia. Simulations showed that after 12 wks of VISMO, 6 wks of I/D could lead to complete resolution of all grades of muscle spasm in 80% of pts and 4 wks of I/D could lead to significant improvement without grade ?2 muscle spasms in 95% of pts. For dysgeusia/ageusia, a 12-wk I/D could lead to complete resolution of all grades of dysgeusia/ageusia in 80% of pts, and a 6-wk I/D could lead to a significant improvement without grade ?2 dysgeusia/ageusia in 95% of pts. Conclusion: Longitudinal PK/PD modeling indicates that 4 to 6 wks and 6 to 12 wks of treatment I/D may lead to significant improvement and complete resolution of ADRs typically associated with VISMO, respectively. Such data may enable the optimization of VISMO treatment interruption.
CATEGORY VII: Emerging translational data – impact on future therapy
High-Dose (HD) IL-2 Immunotherapy for Metastatic Melanoma (mM) in the Era of Checkpoint Inhibitors and Targeted Therapies: Analysis of the PROCLAIM 2007-2012 National Registry
Gregory A. Daniels,1 Michael K. Wong,2 Howard L. Kaufman,3 David F. McDermott,4 Michael A. Morse,5 Sandra Aung6
1University of California, San Diego; 2University of Southern California; 3Rutgers Cancer Center; 4Beth Israel Hospital; 5Duke University Medical Center; 6Prometheus Laboratories Inc.
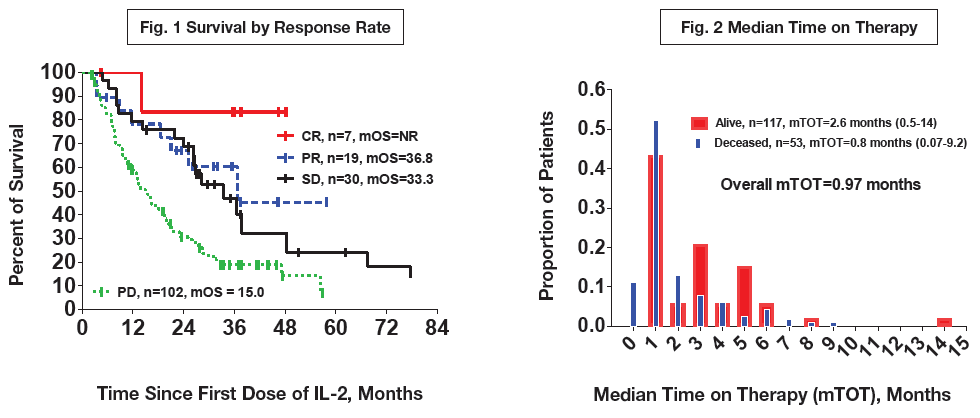
Background: HD IL-2 has been reported to have an overall response rate (ORR) for mM of 16% and a median OS of 11.4 months (Atkins, 1999); however, the studies that led to its regulatory approval are >15 years old and were performed in an era predating checkpoint inhibition and targeted therapies. Methods: The PROCLAIMSM registry (www.proclaimregistry.com), an HD IL-2 observational database currently with over 38 participating sites, consists of a retrospective cohort (treated 2007-2012) informing an ongoing prospective cohort (>650 patients). We report on the retrospective mM subjects (n=170, 13 sites) with survival status determined as of April 2014 and a median follow-up of 37 months. Sites were encouraged to enroll patients sequentially. Inclusion criteria required that patients have received at least 1 dose of HD IL-2. Results: The ORR for mM in the database, 16%, was similar to the historical rates. All 170 subjects were accounted for, 117 were deceased and 53 were known to be alive. Median OS (mOS) for all patients was 20 months, for patients with complete response (CR) mOS was not reached, for patients with partial response (PR), stable disease (SD), and progressive disease (PD) the mOS was 36.8, 33.3, and 15 months, respectively (Figure 1). The median time on therapy (mTOT) was 1 month (Figure 2), with response typically determined 6-8 weeks after treatment. Comparison of patients who received HD IL-2 as 1st or 2nd line (n=128 and 42, respectively) showed no significant difference in OS between these patients. No deaths due to IL-2–related toxicity were reported in the retrospective cohort. Conclusion: The PROCLAIM registry documents an improvement in OS for patients treated with HD IL-2 as compared to the historical reference standards. Response to HD IL-2 traditionally defined as CR or PR, according to these data, should also include SD, which can be very durable. The observation that 1st and 2nd line HD IL-2 possessed similar OS raises intriguing hypotheses about the sequencing of immunotherapy and targeted therapy in mM and the utility of IL-2 as a salvage option – all of which are currently under examination in the prospective database.