Case Report
 A 64-year-old woman was examined at our emergency department after a 6-month period of suffering from progressive fatigue, anorexia, significant weight loss, and abdominal pain. She had a medical history of active smoking and mixed anxiety-depressive disorder treated with bromazepam and escitalopram. The patient had a performance status of 2, and the physical examination showed significant hepatomegaly and pallor. The initial laboratory test showed increases in ALT, AST, and ALP, microcytic-hypochromic anemia, thrombocytosis, and leucocytosis. An abdominal ultrasound was performed showing multiple liver metastases.
A 64-year-old woman was examined at our emergency department after a 6-month period of suffering from progressive fatigue, anorexia, significant weight loss, and abdominal pain. She had a medical history of active smoking and mixed anxiety-depressive disorder treated with bromazepam and escitalopram. The patient had a performance status of 2, and the physical examination showed significant hepatomegaly and pallor. The initial laboratory test showed increases in ALT, AST, and ALP, microcytic-hypochromic anemia, thrombocytosis, and leucocytosis. An abdominal ultrasound was performed showing multiple liver metastases.
Additional laboratory tests revealed hypoalbuminemia of 2.2 g/dL and an increase in several blood tumor markers, including CEA, CA19-9, CA125, and alpha-fetoprotein. The CT scan (Figure 1, panel A) showed a large hepatomegaly due to diffuse liver metastases and a possible mass within the gastric antrum. An upper endoscopy was performed displaying the mentioned gastric antrum mass. Several endoscopic biopsies were performed, and the pathology report confirmed the diagnosis of a poorly differentiated, intestinal Lauren type gastric adenocarcinoma of the antral mucosa. A fine-needle aspiration of liver lesions confirmed the presence of adenocarcinoma as well. Finally, fluorescence in situ hybridization (FISH) analysis showed HER2-positive status.
[caption id="attachment_3334" align="alignleft" width="572"] The CT scan (Figure 1, panel A) showed a large hepatomegaly due to diffuse liver metastases and a possible mass within the gastric antrum. Figure 1, panel B shows the maintained significant radiological response at the liver metastases after 1 year of treatment (24 treatment courses)[/caption]
The CT scan (Figure 1, panel A) showed a large hepatomegaly due to diffuse liver metastases and a possible mass within the gastric antrum. Figure 1, panel B shows the maintained significant radiological response at the liver metastases after 1 year of treatment (24 treatment courses)[/caption]With the diagnosis of a metastatic HER2-positive gastric adenocarcinoma, we initiated first-line treatment with a combination of continuous infusion 5-fluorouracil (5-FU) and oxaliplatin IV (the FOLFOX6 scheme) every 2 weeks, plus weekly trastuzumab (4 mg/kg loading dose followed by 2 mg/kg weekly). The first radiological evaluation after 4 months of treatment showed partial response by RECIST. Figure 1, panel B shows the maintained significant radiological response at the liver metastases after 1 year of treatment (24 treatment courses). Concurrently, the patient experienced a notable improvement in the initial symptoms, achieving a performance status of 0 with a grade 1 oxaliplatin-induced peripheral sensitive neuropathy as the only significant treatment toxicity.
Targeting the Human Epidermal Growth Factor Receptor Type 2 (HER2) Pathway
The HER2 receptor is codified by the c-erbB-2 gene, which was first described in 1985 as a distinct protooncogene but related to the c-erbB-1 gene that codifies the human epidermal growth factor receptor type 1 (HER1 or EGFR) in human breast and salivary gland adenocarcinoma.1,2 Further studies showed that the HER2 gene was also found amplified in gastric adenocarcinoma and located on chromosome 17 at q21.3 Similar to other ERBB family receptors (EGFR, HER3, and HER4), HER2 is composed of extracellular, transmembrane, and intracellular regions (Figure 2).
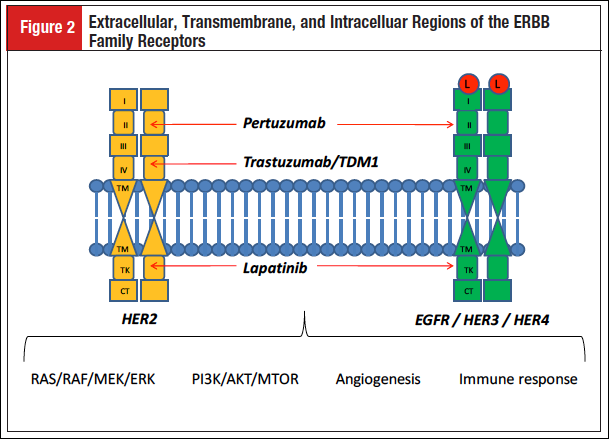
Four domains form the extracellular region. Domains I and III are responsible for the ligand-binding activity, but in HER2 this activity is missing due to the constitutive activity of the receptor, which does not need any ligand binding. This characteristic explains why HER2 is the preferred partner to form homodimers and heterodimers with other ERBB receptors, a dimerization process mediated mainly by the extracellular domain II. Finally, the intracellular region with the tyrosine kinase domain is responsible for the activation of the downstream signaling pathways, which regulates proliferation, cell survival, and angiogenesis.4
[caption id="attachment_3336" align="alignleft" width="650"]
 The Table summarizes the anti-HER2 agents developed to date for the treatment of gastrointestinal tumors.[/caption]
The Table summarizes the anti-HER2 agents developed to date for the treatment of gastrointestinal tumors.[/caption]Several anti-HER2–targeted agents have been developed, mainly in breast cancer research. With focus on gastrointestinal malignancies, the main development of anti-HER2 therapies has been linked to gastroesophageal cancer. The Table summarizes the anti-HER2 agents developed to date for the treatment of gastrointestinal tumors.
Trastuzumab is a humanized IgG1 monoclonal antibody that binds to the extracellular domain IV of HER2, blocking the cleavage of the receptor, avoiding the dimerization, and inhibiting the downstream intracellular signaling activity, including the RAS/RAF/MEK/ERK, the PI3K/AKT/mTOR, and the angiogenesis pathways. In addition, trastuzumab possesses a direct antibody-dependent toxicity.5 Lapatinib is an oral drug with a low molecular weight that inhibits, in a reversible manner, the activity of the intracellular tyrosine kinase domain of the EGFR and HER2 receptors. Lapatinib competes with adenosine triphosphate in the tyrosine kinase domain, blocking the activation of both HER2 and EGFR, and inhibits the downstream signaling pathways of both receptors.6 Pertuzumab is a humanized IgG1 monoclonal antibody that targets domain II of the extracellular region of HER2 as well as EGFR, HER3, and HER4. Pertuzumab blocks the formation of both homodimers and heterodimers of the previous receptors, inhibiting their activation.7 Trastuzumab emtansine (TDM1) is a novel antibody-drug conjugate composed of the monoclonal antibody trastuzumab linked to the cytotoxic drug DM1 (a derivative of maytansine). TDM1 binds to HER2 and gets into the cell by endocytosis of the TDM1-HER2 complex. The proteolytic degradation of the antibody inside the lysosome releases the active DM1, promoting microtubule damage and consequently cell death. Moreover, DM1 action does not interfere with the antitumor effect of trastuzumab.8
Despite the existence of a predictive biomarker, the HER2 protein overexpression or its gene amplification, patients treated with HER2-directed therapy will eventually progress due to resistance to HER2 therapies.9 Some of the proposed mechanisms of resistance to TDM1, which are partially shared with trastuzumab, are low HER2 expression, poor internalization of the HER2-TDM1 complex, defective intracellular transport of the complex, inefficient lysosomal degradation of the complex, higher rate of the complex recycling, masking of the trastuzumab-binding epitope in HER2, higher expression of the truncated p95 HER2 form, MDR1 and other drug efflux pump effects, activation of the neuregulin-HER3 signaling, or presence of altered or mutant tubulins.10 Lapatinib resistance can be explained by the activation of alternative tyrosine kinase pathways (HER3, MET, AXL), intracellular kinases (Akt, mTOR, p70S6K, Src, PTK6), multiple kinase adaptation (JAK, FGFR, DDR1, PIM1, FAK, LIMK), ligand-induced rescue (HRG, NRG1), or the estrogen receptor pathway. In addition, lapatinib resistance can also be explained by the presence of HER2 mutation (T798I) or gene amplifications different from HER2 (NIBP).11 The detailed description of the possible mechanisms of resistance to pertuzumab treatment is a pending issue to be resolved in the near future.
Gastroesophageal Cancer
Overexpression or amplification of HER2 is the unique predictive biomarker of response in gastroesophageal cancer and plays a key role in tumorigenesis in a subset of approximately 15% of patients, although HER2 positivity differ from one report to another due to differences in the analyzed specimens or clinical and pathologic features.12-14 Initially, HER2 positivity was linked to a worse patient survival, but with the incorporation of HER2-targeted therapy, HER2-positive patients treated with anti-HER2 agents have a better prognosis when compared with HER2-negative patients.15
After the approval for the treatment of patients with breast cancer, trastuzumab received approval for the treatment of gastroesophageal cancer based on the results from the ToGA trial, becoming the first targeted agent approved for the treatment of advanced gastroesophageal cancer.16 This randomized phase 3 trial randomized 594 patients with advanced gastroesophageal adenocarcinoma to receive trastuzumab or placebo combined with cisplatin plus a fluoropyrimidine chemotherapy, either 5-FU or capecitabine. Only centrally assessed HER2-positive patients could be enrolled in the trial; patients were considered HER2 positive if samples were scored as 3+ on immunohistochemistry (IHC) or were fluorescence in situ hybridization (FISH) positive with a HER2/CEP17 ratio of ≥2. The patients treated in the trastuzumab arm achieved a statistically significant benefit in both overall response rate (ORR) (odds ratio [OR] 1.7; 95% CI, 1.22-2.38; P = .0017), progression-free survival (PFS) (hazard ratio [HR] 0.71; 95% CI, 0.59-0.85; P = .0002), and overall survival (OS) (HR 0.74; 95% CI, 0.60-0.91; P = .0046), with no difference in the overall rate of adverse events. In addition, a more marked OS benefit was observed in the patients with high HER2 expression (IHC3+ or IHC2+/FISH+), with a median survival of 16 months (HR 0.65; 95% CI, 0.51-0.83) that was never achieved in previous trials in which median survival was consistently set below 12 months. Furthermore, the addition of trastuzumab to chemotherapy produced a benefit in quality of life when compared with chemotherapy alone and showed a favorable cost-effectiveness ratio.17,18 Trastuzumab has also demonstrated efficacy without toxicity concerns when combined with other chemotherapy agents such as oxaliplatin, S1, or cisplatin monotherapy.19-21
Lapatinib activity in gastric adenocarcinoma was tested in the TyTAN study.22 This phase 3 trial randomized 261 patients with advanced gastric cancer to receive paclitaxel with or without lapatinib in the secondline setting. Eligible patients were HER2 positive as defined by a FISH+ test result. The combination in the experimental arm did not show a significant benefit in neither PFS (HR 0.85; 95% CI, 0.63-1.13; P = .2441) nor OS (HR 0.84; 95% CI, 0.64-1.11; P = .1044). However, a statistically significant benefit was observed in ORR (27% vs 9%; OR 3.85; 95% CI, 1.80-8.87; P = .001) as well as in the HER2 IHC3+ population for both PFS (HR 0.54; 95% CI, 0.33-0.90; P = .0101) and OS (HR 0.59; 95% CI, 0.37-0.93; P = .0176), with no significant safety concerns. Although the combination of lapatinib plus paclitaxel may be active as a second-line treatment for the FISH+ or IHC3+ subset of patients with HER2-positive advanced gastric cancer, this treatment modality is not currently approved by regulatory agencies. Lapatinib monotherapy and the combination of lapatinib and capecitabine were explored in randomized phase 2 trials in advanced gastric cancer, failing to demonstrate sufficient activity, and with an unfavorable toxicity profile.23,24
The optimal dose of pertuzumab combined with chemotherapy was set at 840 mg every 3 weeks in a phase 2 trial with HER2-positive advanced gastric cancer.25 Ongoing trials are testing the activity of pertuzumab and TDM1 in the first- and second-line setting, respectively, for HER2-positive advanced gastroesophageal adenocarcinoma. We are eagerly awaiting the results from these 2 large randomized phase 3 trials, which may change the standard clinical practice for patients with gastroesophageal cancer in the near future. Finally, the authorized incorporation of HER2-directed therapy in locally advanced gastroesophageal cancer is warranted due to the activity shown in the metastatic setting.26,27
Colorectal cancer
Colorectal cancer (CRC) is the most common gastrointestinal tumor, and targeted therapy in CRC has been focused on the development of anti-VEGFR (vascular endothelial growth factor receptor) and anti-EGFR drugs. HER2 positivity has been assessed in several series of CRC patients. One of the largest series with 1645 surgically removed primary CRCs detected only 1.6% of the samples as being HER2 positive.28 However, higher rates of HER2 positivity (26%) were observed when limiting the test to KRAS/BRAF wild-type CRC.29 HER2 overexpression or amplification is considered one of the mechanism of resistance to EGFR inhibitors in CRC patients.30 More recently, HER2 somatic activating mutations (S310F, L755S, V777L, V842I, and L866M) have been shown to cause resistance to EGFR inhibitors in CRC that can be reversed with HER2-directed therapy.31 In addition, HER3 overexpression is as high as 69% in CRC, with a good correlation to HER2 overexpression and gene amplification.32
Some publications have reported dramatic responses in HER2-positive patients with CRC treated with anti-HER2 therapies.33 Lapatinib was studied in a phase 2 trial with chemorefractory CRC patients. In this trial, 29 patients were enrolled, independent of their HER2 status, to be treated with the combination of lapatinib and capecitabine. The median survival was poor at 6.8 months, and there were no objective responses, but 41% of the patients achieved stable disease, probably due to the nonmolecularly selected population.34 Pertuzumab was tested with promising activity in a phase 1/2 trial combined with cetuximab in refractory CRC patients, but dose-limiting skin and gastrointestinal toxicities did not allow the trial to continue.35 The first phase 2 trial conducted with HER2-directed therapy tested the combination of trastuzumab plus irinotecan in 9 HER2-positive patients with CRC in the second-line setting. HER2 positivity was assessed by IHC overexpression and was detected in 8% of the 138 screened patients. The treatment showed encouraging activity with response in 5 of the 7 evaluable patients.36 Finally, it is worth noting the results of the best-conducted trial in HER2-positive CRC patients treated with HER2-targeted therapy, the HERACLES trial. This study was a phase 2 trial in which patients with KRAS wild-type advanced CRC, progressing on all chemotherapy agents and anti-EGFR inhibitors, were treated with a combination of lapatinib and trastuzumab based on preliminary preclinical data. Of the 646 patients with KRAS wild-type CRC, only 5.4% were considered HER2 overexpressed or amplified and selected for enrollment. The trial was positive with an ORR of 34% and a time to progression of 5.4 months.37 These encouraging results warrant further investigation, and new agents such as pertuzumab or TDM1 should be assessed for the treatment of patients with CRC.
Pancreatic Cancer
Pancreatic cancer is a highly lethal disease for which no targeted therapies have been approved to date. Chemotherapy achieves a poor outcome in the metastatic setting, with a median survival of less than 12 months. Anti-HER2 therapy may be an option for selected patients. In a large cohort of 469 patients with pancreatic ductal adenocarcinoma, HER2 overexpression was detected in 7%, while gene amplification was observed in 2% of the patients.38
First-line treatment with lapatinib and gemcitabine was tested in a phase 2 trial in patients with advanced pancreatic adenocarcinoma. The trial closed after enrolling 29 patients due to futility analysis. The ORR was 10%, with a median survival of 4 months.39 The combination of trastuzumab and cetuximab was explored in a phase 1/2 trial in pancreatic adenocarcinoma after gemcitabine failure. The study was negative as no objective responses were observed, and substantial skin toxicity limited the treatment compliance. The median PFS and OS were 1.8 and 4.6 months, respectively.40 It is noteworthy that the previous trials were conducted in a nonselected population concerning HER2 status. An early phase 2 trial in patients with advanced pancreatic cancer tested the combination of trastuzumab plus gemcitabine in a HER2 overexpressed population. The median survival of 7 months did not differ much from that achieved with gemcitabine monotherapy in historical controls.41 The combination of trastuzumab and capecitabine was used as first-line treatment in a phase 2 trial in patients with HER2-positive advanced pancreatic adenocarcinoma. Patients were considered HER2-positive if IHC3+ or IHC2+/FISH+, with a detection rate of 11% in the 212 patients screened. The study was closed prematurely because of slow accrual, and the results were considered negative, with no improvement in 3-month PFS (23.5%) or median OS (6.9 months).42 Further studies with novel anti-HER2 treatments, such as TDM1 or pertuzumab, need to explore the role of HER2-targeted therapy in pancreatic cancer. However, the low rate of HER2-positive patients may be a limitation for the accrual in future trials.
Hepatobiliary Tumors
Hepatobiliary tumors comprise several tumor types, which are almost always mixed in clinical trials, with the consequent difficulty in conducting high-quality studies. No targeted therapy is currently used for this group of tumors, with the exception of sorafenib in hepatocellular carcinoma and gemcitabine with platinum in biliary tract carcinomas as the standard of care. A retrospective analysis comprising 236 cholangiocarcinoma samples from an Asian population explored the expression of EGFR, VEGF, and HER2 as measured by IHC testing. The authors found a HER2-positive expression of 8.5% (11 patients) among the extrahepatic cholangiocarcinoma patients, while only 0.9% (1 patient) had HER2 expression in the intrahepatic cholangiocarcinoma group.43 In another retrospective study, this time in a European population, 124 samples from patients with advanced biliary tract cancer (BTC) were analyzed for the expression of EGFR and HER2. Overall, 39.2% of the samples were considered EGFR positive as defined by an IHC result of 2+ or 3+. HER2 expression was observed in 18% (IHC2+) and 3% (IHC3+), while HER2 gene amplification (FISH+) was seen in 5% of patients.44 However, recent studies describe higher rates of HER2-positive cases in the subgroup of patients with gallbladder carcinoma (GBC). In this report, HER2 stained 2+ in 20% and 3+ in 13% of patients, and the overexpression of HER2 was associated with a worse survival when compared with the absence of HER2 expression.45
Lapatinib was the first anti-HER2 agent tested in hepatobiliary tumors because of its capacity to block both EGFR and HER2. A phase 2 trial was conducted in 17 patients with biliary tree carcinoma and 40 patients with hepatocellular carcinoma (HCC). Molecular selection by EGFR or HER2 expression was not performed. Lapatinib monotherapy, although well tolerated, showed only 2 objective responses in the HCC group, and stabilization was achieved in 17 patients in the 2 groups. The median PFS and OS were 1.8 and 5.2 months, respectively, for biliary tree carcinoma, whereas it was slightly superior in HCC with a PFS of 2.3 months and OS of 6.2 months. The occurrence of skin rash was associated with a better survival outcome.46 A second attempt with lapatinib monotherapy was made in another phase 2 trial with BTC patients, again in a nonmolecularly selected population. Although few conclusions can be drawn as the trial was stopped after 9 patients were enrolled, results similar to the previous trial were achieved, with a low ORR (0%), an interesting stabilization rate (50%), a median PFS of 2.6 months, and an OS of 5.1 months, with no additional toxicity concerns.47 Nevertheless, dramatic objective responses have been reported in patients with BTC harboring HER2 amplification when treated with trastuzumab.48 Overexpression of HER2 and/or HER3 can be observed in one-third of BTC patients, with rates of gene amplification in 17% to 27% of the patients, a scenario in which pertuzumab has shown activity in cell line preclinical trials.49 More recent studies retrospectively reviewed the activity of anti-HER2–targeted therapy (including trastuzumab, lapatinib, or pertuzumab) for advanced BTC with HER2 overexpression or amplification. Among the 5 cholangiocarcinoma patients, no objective responses were achieved, with a high incidence of HER2 gene mutations in this population. However, in the 9 patients with GBC, 1 complete response, 3 partial responses, and 4 stabilizations were achieved.50 To sum up, there is sufficient evidence to think that HER2-targeted therapy may be active in the subset of HER2-positive patients with hepatobiliary tumors. It seems to merit further investigation, but again, the low incidence of these tumors is a challenge for the conduction of clinical trials.
Conclusion
Targeted therapy against HER2 is considered a milestone in the treatment of patients with HER2-positive breast and gastroesophageal cancer. The identification of HER2 as a predictive biomarker and the incorporation of HER2-directed therapy in the cancer armamentarium for HER2-positive tumors have achieved a huge benefit in survival for these patients that is far above that obtained with cytotoxic chemotherapy alone. In the case report, the addition of trastuzumab to a FOLFOX backbone chemotherapy scheme produced a significant objective ORR and a prolonged PFS. Novel anti-HER2 agents (pertuzumab, lapatinib, and TDM1) have been developed to increase trastuzumab efficacy and overcome trastuzumab resistance, but in the end, tumors find new ways to progress on these therapies.
Gastroesophageal cancer was the first gastrointestinal tumor in which HER2 overexpression or amplification was established as a predictive biomarker for response to trastuzumab treatment. At present, HER2 status identification is mandatory in gastroesophageal tissue samples, and trastuzumab treatment has become the standard of care for HER2 metastatic disease. In pancreatic cancer or hepatobiliary tumors, the low rate of HER2 positivity and the low incidence of these malignancies are some of the challenges to properly develop good quality clinical trials to test HER2-directed therapies. Nevertheless, the encouraging results of trastuzumab treatment in CRC warrants further clinical trials in order to incorporate, potentially, anti-HER2 therapies into the armamentarium.
References
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974-976.
- Semba K, Kamata N, Toyoshima K, et al. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985;82:6497-6501.
- Fukushige S, Matsubara K, Yoshida M, et al. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955-958.
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354.
- Sanford M. Trastuzumab: a review of its use in HER2-positive advanced gastric cancer.Drugs. 2013;73:1605-1615.
- Frampton JE. Lapatinib: a review of its use in the treatment of HER2-overexpressing, trastuzumab-refractory, advanced or metastatic breast cancer. Drugs. 2009;69:2125-2148.
- McCormack PL. Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer. Drugs. 2013;73:1491-1502.
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280-9290.
- Thery JC, Spano JP, Azria D, et al. Resistance to human epidermal growth factor receptor type 2-targeted therapies. Eur J Cancer. 2014;50:892-901.
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209.
- D’Amato V, Raimondo L, Formisano L, et al. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat Rev. 2015;41:877-883.
- Park YS, Hwang HS, Park HJ, et al. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: which scoring system should we use? Hum Pathol. 2012;43:413-422.
- Pirrelli M, Caruso ML, Di Maggio M, et al. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig Dis Sci. 2013;58:397-404.
- Huang D, Lu N, Fan Q, et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One. 2013;8:e80290.
- Shitara K, Yatabe Y, Matsuo K, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer. 2013;16:261-267.
- Bang YJ, Van Cutsem E, Feyereislova A, et al; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697.
- Satoh T, Bang YJ, Gotovkin EA, et al; ToGA Trial Investigators. Quality of life in the trastuzumab for gastric cancer trial. Oncologist. 2014;19:712-719.
- Shiroiwa T, Fukuda T, Shimozuma K. Cost-effectiveness analysis of trastuzumab to treat HER2-positive advanced gastric cancer based on the randomised ToGA trial. Br J Cancer. 2011;105:1273-1278.
- Ryu MH, Yoo C, Kim JG, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51:482-488.
- Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110:1163-1168.
- Grávalos C, Gómez-Martín C, Rivera F, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol. 2011;13:179-184.
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049.
- Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610-2615.
- Lorenzen S, Riera Knorrenschild J, Haag GM, et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur J Cancer. 2015;51:569-576.
- Kang YK, Rha SY, Tassone P, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660-666.
- ClinicalTrials.gov identifier: NCT01774786
- ClinicalTrials.gov identifier: NCT01641939
- Ingold Heppner B, Behrens HM, Balschun K, et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111:1977-1984.
- Herreros-Villanueva M, Rodrigo M, Claver M, et al. KRAS, BRAF, EGFR and HER2 gene status in a Spanish population of colorectal cancer. Mol Biol Rep. 2011;38:1315-1320.
- Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508-523.
- Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832-841.
- Seo AN, Kwak Y, Kim WH, et al. HER3 protein expression in relation to HER2 positivity in patients with primary colorectal cancer: clinical relevance and prognostic value. Virchows Arch. 2015;466:645-654.
- Disel U, Germain A, Yilmazel B, et al. Durable clinical benefit to trastuzumab and chemotherapy in a patient with metastatic colon adenocarcinoma harboring ERBB2 amplification. Oncoscience. 2015;2:581-584.
- Frank D, Jumonville A, LoConte NK, et al. A phase II study of capecitabine and lapatinib in advanced refractory colorectal adenocarcinoma: a Wisconsin Oncology Network study. J Gastrointest Oncol. 2012;3:90-96.
- Rubinson DA, Hochster HS, Ryan DP, et al. Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients. Invest New Drugs. 2014;32:113-122.
- Ramanathan RK, Hwang JJ, Zamboni WC, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22:858-865.
- Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): The HERACLES trial. J Clin Oncol. 2015;33(suppl). Abstract 3508.
- Chou A, Waddell N, Cowley MJ, et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5:78.
- Safran H, Miner T, Bahary N, et al. Lapatinib and gemcitabine for metastatic pancreatic cancer. A phase II study. Am J Clin Oncol. 2011;34:50-52.
- Assenat E, Azria D, Mollevi C, et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: results of the “THERAPY” phase 1-2 trial. Oncotarget. 2015;6:12796-12808.
- Safran H, Iannitti D, Ramanathan R, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004; 22:706-712.
- Harder J, Ihorst G, Heinemann V, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038.
- Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425.
- Harder J, Waiz O, Otto F, et al. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511-4517.
- Roa I, de Toro G, Schalper K, et al. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014;7:42-48.
- Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777-783.
- Peck J, Wei L, Zalupski M, et al. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology. 2012;82:175-179.
- Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. J Clin Oncol. 2012;30:e271-e273.
- Kawamoto T, Ishige K, Thomas M, et al. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. J Gastroenterol. 2015;50:467-479.
- Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58.

