A mutation of the epidermal growth factor
receptor (EGFR), known as EGFR variant III (EGFRvIII), is expressed in a significant proportion of glioblastoma tumors and is linked to poor long-term survival. Unlike unmutated EGFR, EGFRvIII has not been detected at a significant level in normal tissues; therefore, targeting of this tumor-specific molecule is not likely to impact healthy tissues. A number of immunotherapeutic approaches are in development that target EGFRvIII in glioblastoma.

The EGFR plays a role in cellular processes such as migration, differentiation, and apoptosis.1
The EGFR is often amplified in glioblastomas and provides a potential therapeutic target; however, normal tissues also express EGFR, and targeting all EGFRs can lead to unintended damage to normal tissue.2 One specific, spontaneously occurring EGFR mutation,
EGFRvIII, is found in approximately 30% of glioblastomas but is rarely found in normal brain tissue, so targeting of EGFRvIII is not likely to impact normal tissue.3-5 This constitutively active mutant receptor leads to unregulated growth, survival, invasion, and angiogenesis in cells that express it.6-10 For the small subset of patients with glioblastoma who survive 1 year or longer after diagnosis, the expression of EGFRvIII is also an independent negative prognostic indicator of survival.11 Because of this, targeting EGFRvIII represents a very promising therapeutic strategy for patients with glioblastoma, and a number of potential immunotherapies are in development (Table 1).

Rindopepimut
Rindopepimut (CDX-110) is a cancer vaccine with potential antineoplastic activity consisting of an
EGFRvIII-specific peptide conjugated to the nonspecific immunomodulator keyhole limpet hemocyanin (KLH). Vaccination with rindopepimut may elicit a cytotoxic T-lymphocyte immune response against tumor cells expressing EGFRvIII.
Three phase 2 trials of rindopepimut (ACTIVATE, ACT II, and ACT III) have been completed in newly diagnosed EGFRvIII-positive glioblastoma and have shown consistent improvement in overall survival (Figure 1).12 These long-term results represent a substantial survival benefit in comparison with independent control data sets, suggesting that rindopepimut is providing survival beyond what is historically seen in EGFRvIII-expressing glioblastoma patients—a group that typically has more aggressive disease associated with a worse prognosis than the general glioblastoma patient population.12

In ACTIVATE (N = 18), patients with newly diagnosed EGFRvIII-expressing glioblastomas having a gross total resection (>95%), Karnofsky performance status ?80, and not exhibiting radiographic progression following external beam radiotherapy and concurrent temozolomide therapy were treated with rindopepimut vaccine and granulocyte-macrophage colony-stimulating factor (GM-CSF), which has been shown to enhance immune responses to vaccination.13,14 At the time of tumor recurrence, 82% (95% CI, 48%-97%) of patients had lost EGFRvIII expression after receiving an
EGFRvIII-targeted vaccination (P <.001). This suggested that a specific effect of the vaccine was to eliminate EGFRvIII-expressing tumor cells, which have been associated with a worse
prognosis.13
ACT II (N = 22) was designed to assess the efficacy of rindopepimut with standard adjuvant temozolomide 200 mg/m2 for the first 5 days of a 28-day cycle plus GM-CSF versus temozolomide 100 mg/m2 for the first 21 days of a 28-day cycle plus GM-CSF.15 The patients who received the prolonged temozolomide dosing developed more severe lymphopenia; however, there was an even more robust serum immunity to
EGFRvIII.
In ACT III, rindopepimut and standard adjuvant temozolomide chemotherapy were administered to 65 patients with newly diagnosed EGFRvIII-expressing glioblastoma after gross total resection and chemoradiation, and results confirmed, in a multicenter setting, the preliminary results seen in previous phase 2 studies.16
Ongoing Rindopepimut Studies
Rindopepimut is currently being studied in 2 clinical trials in EGFRvIII-positive glioblastoma: an international phase 3 study called ACT IV (NCT01480479) in newly diagnosed glioblastoma and a phase 2 study called ReACT (NCT01498328) in recurrent glioblastoma.
ACT IV is a randomized, double-blind, controlled phase 3 study investigating the efficacy and safety of the addition of rindopepimut plus GM-CSF (given as a vaccine adjuvant) to the current standard of care, temozolomide, in patients with newly diagnosed EGFRvIII-positive glioblastoma who have had surgery and radiation plus treatment with temozolomide. Patients are randomly assigned to receive rindopepimut or KLH (used as a control), each along with temozolomide. KLH, an immunogenic carrier protein, is one of the ingredients in the rindopepimut vaccine, but it is not expected to have significant anticancer activity when given alone at this low dose. KLH was selected as a control for this study because of its ability to generate an injection site reaction similar to that observed with the rindopepimut vaccine, which improves the blinding of the study. Patients are treated until disease progression, and all patients are followed for survival. This trial is currently ongoing but is not enrolling patients.
ReACT is an ongoing phase 2 study designed to determine if adding rindopepimut to the standard of care for relapsed glioblastoma, bevacizumab, improves the outcomes for patients with EGFRvIII-positive recurrent glioblastoma.17 The study includes 3 groups:
- Group 1 (n = 72): patients with relapsed glioblastoma who have never been treated with bevacizumab. These patients are randomly assigned to receive either rindopepimut/GM-CSF or KLH (administered as a control), each along with bevacizumab
- Group 2 (n = 25): patients refractory to bevacizumab. These patients receive rindopepimut along with bevacizumab in a single treatment arm
- Group 2C (C = confirmatory): this expansion of group 2 will consist of up to 75 patients who will receive rindopepimut plus bevacizumab in a single treatment arm.
The primary end point of the study is progression-free survival at 6 months (ie, the percentage of patients alive without progression at 6 months). All patients will be treated until disease progression and will be followed for survival.
Interim results from the ReACT study were presented in November 2014 at the 4th Quadrennial Meeting of the World Federation of Neuro-Oncology held in conjunction with the 19th Annual Meeting of the Society for Neuro-Oncology. At the time of the meeting, results showed that, among 72 bevacizumab-naive patients who were randomized to receive either rindopepimut plus bevacizumab (n = 35) or control plus bevacizumab
(n = 37), a statistically significant benefit in overall survival (OS) was seen in favor of the patients treated with rindopepimut (hazard ratio 0.47; P = .0208). Median OS was 12.0 months for the rindopepimut plus bevacizumab group and 8.8 months for the control group (Table 2). At the time of the meeting, 27% of patients treated with rindopepimut were progression free compared with 11% of the control patients (P = .048). Seven of 29 patients (24%) evaluable for response in the rindopepimut group experienced a confirmed objective response versus 5 of 30 patients (17%) evaluable for response in the control group. In addition, 74% of patients in the rindopepimut group had stable disease or better for >2 months versus 57% in the control group.17
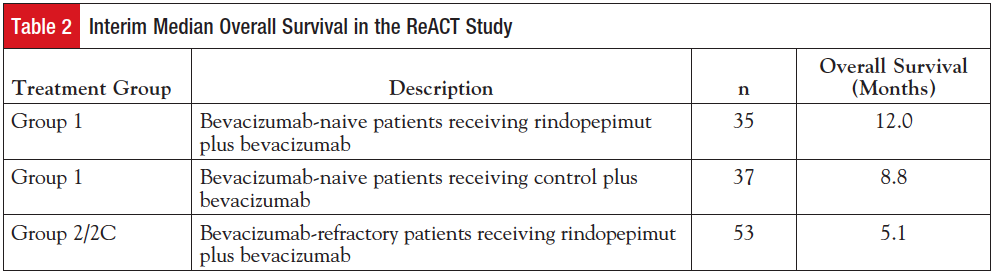
Results were also presented for 53 patients (25 patients in group 2; 28 patients in group 2C) refractory to bevacizumab who were receiving rindopepimut along with bevacizumab in the study. The median OS was 5.1 months (95% CI, 3.2-6.5) in these heavily pretreated, refractory EGFRvIII-positive patients (Table 2). In comparison, a review of the literature assessing survival in recurrent bevacizumab-experienced patients across 8 independent studies suggests a weighted average survival of 3.6 months (range, 2.6-5.8 months) in all-comers. Forty-six percent of patients in group 2/2C were alive at 6 months. Nineteen percent of patients had stable disease or better for >2 months (range, 2.8-16.5 months).17
ADU-623
The ADU-623 vaccine is a live-attenuated, double- deleted strain of the Gram-positive bacterium Listeria monocytogenes that targets dendritic cells and expresses 2 cancer-specific antigens, EGFRvIII and NY-ESO-1 (a cancer/testis antigen). The vaccine promotes a potent innate immune response as well as an adaptive immune response and targets not only EGFRvIII-expressing tumor cells but also those expressing NY-ESO-1.18 ADU-623 is being investigated in a phase 1 study that will enroll up to 38 patients with high-grade gliomas (including glioblastomas) who have previously been treated with standard-of-care therapy. The study will evaluate 3 dose levels of ADU-623 with the primary goal of establishing the safety of the immunotherapy and to determine the optimal dose. The trial will also evaluate the patients’ tumor responses and immune responses to the ADU-623 immunotherapy.19
Anti-EGFRvIII Chimeric Antigen Receptor T Cells
Chimeric antigen receptors (CARs) represent an emerging technology that combines the variable region of an antibody with T-cell signaling moieties and can be genetically expressed in T cells to mediate potent antigen-specific activation.20 CAR T cells have the potential to eradicate neoplasms by recognizing tumor cells regardless of major histocompatibility complex (MHC) presentation of target antigen or MHC downregulation in tumors, factors that prevent the success of other anticancer treatments.20 Clinical trials using CARs in other types of cancer have demonstrated the potential efficacy of this strategy.20 However, severe adverse events, including patient deaths, have occurred from administration of CAR T cells when directed against tumor antigens simultaneously expressed on normal tissues.21,22 Unlike previous CAR T cells, anti-EGFRvIII CAR T cells have the potential to eliminate tumor cells without damaging normal tissue due to the tumor specificity of its target antigen.20
A phase 1/2 clinical study (NCT01454596) is being conducted by the National Cancer Institute to test the safety and feasibility of administering T cells expressing the anti-EGFRvIII chimeric antigen receptor to patients with malignant gliomas expressing EGFRvIII.23
Antibody-Drug Conjugates
Two therapeutic approaches to cancer therapy are antibodies that specifically bind tumor surface antigens and cytotoxic chemotherapies. Unfortunately, many antibodies lack therapeutic activity, and toxic chemotherapeutic drugs do not selectively localize to tumors, so their systemic drug distribution may result in damage to healthy tissues, and drug dose escalation to therapeutically active levels may be impossible. Because antibodies bind specifically to cells expressing their antigen, they represent ideal “vehicles” or “guided missiles” to deliver cytotoxic drugs directly to the tumor.24 Antibody-drug conjugates (ADCs) combine the potency of cytotoxic agents with the target selectivity of antibodies by chemically linking a cytotoxic payload to an antibody, potentially creating a synthetic molecule that will deliver targeted antitumor therapy that is both safe and efficacious.25
ABT-414
ABT-414 is an ADC consisting of an antibody targeting active EGFR or EGFRvIII conjugated to the cytotoxic agent monomethyl auristatin F. A phase 1 clinical trial (NCT01800695) is being conducted to evaluate the safety and pharmacokinetics of ABT-414 in patients with glioblastoma. There are 3 groups in this study:
- Group A: patients with newly diagnosed glioblastoma and prior surgical resection receive ABT-414 with concurrent radiotherapy and temozolomide
- Group B: patients with newly diagnosed glioblastoma who have completed adjuvant radiation and/or temozolomide therapy or patients with recurrent glioblastoma receive ABT-414 with temozolomide
- Group C: patients with recurrent glioblastoma receive ABT-414 as monotherapy.
Interim results from this phase 1 trial, reported at the 2014 meeting of the Society for Neuro-Oncology, showed that 4 of 12 patients (33%) with measurable disease and EGFR amplification achieved an objective response, including 2 patients who achieved a complete response.26,27 Of the 2 patients who achieved a complete response, one was enrolled in group B and the other in group C. These patients each had disease that recurred after radiation and chemotherapy, a patient population for which effective therapies are very limited. Common adverse events in groups A, B, and C included fatigue, blurred vision, nausea, photophobia, constipation, increased aspartate aminotransferase levels, increased alanine aminotransferase levels, keratitis, thrombocytopenia, dry eye, eye pain, and foreign body sensation in the eye. Grade 3/4 adverse events included keratitis, lymphopenia, and thrombocytopenia. Dose-limiting toxicities occurred at multiple doses and affected the eye (keratitis) and liver.
Based on these results, ABT-414, which was granted orphan drug designation by the Food and Drug Administration and the European Medicines Agency earlier this year, will soon advance to a randomized phase 2 study (NCT02343406) in patients with recurrent glioblastoma.28 There will be 3 groups in this study:
- Group 1 (Experimental): patients with recurrent glioblastoma will receive ABT-414 in combination with temozolomide
- Group 2 (Experimental): patients with recurrent glioblastoma will receive ABT-414 monotherapy
- Group 3A (Active comparator): patients who relapse during treatment with temozolomide or within 16 weeks after the first day of the last cycle of treatment with temozolomide will receive lomustine
- Group 3B (Active comparator): patients who relapse 16 weeks or more after the first day of the last cycle of treatment with temozolomide will receive retreatment with temozolomide.
AMG 595
AMG 595, an ADC consisting of a human monoclonal antibody directed against EGFRvIII, conjugated to the cytotoxic agent mertansine, is in a phase 1, first-in-human, open-label, dose-finding study enrolling patients with recurrent gliomas, including glioblastomas (NCT01475006).29 An immunohistochemical assay developed using a novel EGFRvIII antibody is currently being employed for prospective patient selection in this study.30
References
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354.
- Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expert Rev Vaccines. 2012;11:133-144.
- Wikstrand CJ, Hale LP, Batra SK, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140-3148.
- Humphrey PA, Wong AJ, Vogelstein B, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87:4207-4211.
- Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965-2969.
- Antonyak MA, Moscatello DK, Wong AJ. Constitutive activation of c-Jun N-terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem. 1998;273:2817-2822.
- Moscatello DK, Holgado-Madruga M, Emlet DR, et al. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200-206.
- Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57:1419-1424.
- Li G, Mitra S, Wong AJ. The epidermal growth factor variant III peptide vaccine for treatment of malignant gliomas. Neurosurg Clin N Am. 2010;21:87-93.
- Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087-1098.
- Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462-1466.
- Celldex Therapeutics. Celldex Therapeutics’ rindopepimut demonstrates promising clinical activity in patients with EGFRvIII-positive recurrent glioblastoma at SNO [press release]. http://ir.celldex.com/releasedetail.cfm?ReleaseID=809242. November 24, 2013. Accessed January 30, 2015.
- Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722-4729.
- Babu R, Adamson DC. Rindopepimut: an evidence-based review of its therapeutic potential in the treatment of EGFRvIII-positive glioblastoma. Core Evid. 2012;7:93-103.
- Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate
EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324-333.
- Schuster J, Lai RK, Recht LD. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study [published online January 13, 2015]. Neuro Oncol.
- Celldex Therapeutics. Interim update from randomized phase 2 ReACT study of rindopepimut in recurrent bevacizumab-naive glioblastoma demonstrates statistically significant survival benefit [press release]. http://ir.celldex.com/releasedetail.cfm?Re leaseID=883150. November 15, 2014. Accessed January 30, 2015.
- Aduro BioTech. Aduro BioTech announces initation of a clinical trial of its novel immunotherapy in patients with brain cancer at Providence Cancer Center [press release]. www.aduro.com/news/press-releases/2014/05-05-2014/. May 5, 2014. Accessed February 1, 2015.
- ClinicalTrials.gov. Phase I study of safety and immunogenicity of ADU-623. https://clinicaltrials.gov/ct2/show/NCT01967758. Accessed February 1, 2015.
- Miao H, Choi BD, Suryadevara CM, et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9:e94281.
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843-851.
- Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666-668.
- ClinicalTrials.gov. CAR T cell receptor immunotherapy targeting EGFRvIII for patients with malignant gliomas expressing EGFRvIII. https://clinicaltrials.gov/ct2/show/NCT01454596. Accessed February 1, 2015.
- Panowski S, Bhakta S, Raab H, et al. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6:34-45.
- Leal M, Sapra P, Hurvitz SA, et al. Antibody-drug conjugates: an emerging modality for the treatment of cancer. Ann N Y Acad Sci. 2014;1321:41-54.
- AbbVie. AbbVie presents results from study of ABT-414 in patients with glioblastoma multiforme at the 19th Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology [press release]. http://abbvie.mediaroom.com/2014-11-14-AbbVie-Presents-Results-from-Study-of-ABT-414-in-Patients-with-Glioblas toma-Multiforme-at-the-19th-Annual-Scientific-Meeting-and-Educa
tion-Day-of-the-Society-for-Neuro-Oncology. November 14, 2014. Accessed February 2, 2015.
- Gan HK, Fichtel L, Lassman AB, et al. A phase 1 study evaluating ABT-414 with temozolomide (TMZ) or concurrent radiotherapy (RT) and TMZ in glioblastoma (GBM). Presented at: 19th Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology (SNO); November 13-16, 2014; Miami, Florida. Abstract ET-19.
- ClinicalTrials.gov. ABT-414 alone or ABT-414 plus temozolomide vs. lomustine or temozolomide for recurrent glioblastoma. https://clinicaltrials.gov/ct2/show/NCT02343406?term=NCT02343406&rank=1. Accessed February 1, 2015.
- ClinicalTrials.gov. AMG 595 first-in-human in recurrent gliomas. https://clinicaltrials.gov/ct2/show/NCT01475006. Accessed February 1, 2015.
- Damore MA, Coberly SK, Wakamiya K, et al. An EGFRvIII-specific IHC IUO test for patient selection in AMG 595 phase I trial. J Clin Oncol. 2013;31(suppl). Abstract 2071.

