Targeted treatments have proved to be clinically successful and represent the realization of personalized medicine’s potential. In non–small cell lung cancer (NSCLC), molecularly targeted treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) such as erlotinib and gefitinib have shown dramatic responses in patients with activating mutations.1-3
EGFR is a member of the human epidermal growth factor (HER) or ERBB family, a set of receptor tyrosine kinases that include EGFR (ERBB1), ERBB2, ERBB3, and ERBB4.4 The receptors and their ligands regulate the proliferation of cancerous cells. Elevated levels of EGFR and its ligands are found in early lung cancer and upon progression and metastasis.5 EGFR mutations occur in about 10% of patients with NSCLC but are commonly associated with female nonsmokers of Asian descent.1,6 These mutations occur within exons 18 to 21, which constitute most of the tyrosine kinase binding domain of the receptor.7,8 Exon 19 deletions (del19) and L858R within exon 21 are the 2 most common EGFR mutations, making up about 90% of all EGFR mutations. These mutations alter the receptor’s adenosine triphosphate (ATP) binding pocket by improving its affinity for ATP, resulting in constitutive activation.4-8 Reversible EGFR TKIs, erlotinib and gefitinib, preferentially bind to the receptor’s ATP binding pocket, blocking ATP binding and the dimerization needed for receptor activation.4,6 In contrast, mutations such as exon 20 insertions and in-frame deletions or T790M alter the drug-receptor interaction and revert ATP binding affinity to that of a wild-type receptor.4-7
Despite the incredible responses observed in patients with activating EGFR mutations, resistance invariably occurs. Patients who respond to EGFR TKIs progress after a median of 12 months.9 After treatment with erlotinib or gefitinib, about 50% to 60% of patients develop the exon 20 resistance mutation, T790M.10 Other ways to acquire resistance include the development of mutations affecting downstream targets of EGFR. For example, mutations causing constitutive activation of PI3K lead to gefitinib resistance.11 To combat these resistance mechanisms, second- and third-generation EGFR TKIs have been developed. Second-generation HER TKIs covalently bind to the receptor’s activation domain and inhibit multiple members of the ERBB family.12-15 A recently approved drug that is a part of this class of TKIs is afatinib. Afatinib has primarily been evaluated in patients with EGFR mutation–positive NSCLC as first-line therapy and in those with acquired resistance. This review will present and examine the data from the use of afatinib for the treatment of patients with NSCLC.
Preclinical and Phase 1 Results
Afatinib is an aniline-quinazoline, irreversible EGFR/HER2 inhibitor. In vitro assays demonstrated that afatinib and gefitinib have similar kinase inhibitory activity in fusion constructs with activating L858R mutations.14 In addition, when constructs were L858R/T790M double mutants, afatinib was more potent than gefitinib. Compared against the HER2-specific inhibitors lapatinib and canertinib, afatinib showed similar inhibitory activity. Afatinib was also shown to specifically inhibit EGFR kinase activity when tested against 52 tyrosine and serine/threonine kinases. In human epidermal carcinoma cells (A431) expressing wild-type EGFR, murine NIH-3T3 cells expressing wild-type HER2, breast cancer cells (BT-474), and gastric cancer cells (NCI-N87), afatinib inhibited EGFR or HER2 autophosphorylation at lower concentrations than lapatinib, canertinib, and gefitinib.14 Furthermore, afatinib concentrations needed to inhibit anchorage-independent colony formation of NIH-3T3 cells of 4 different isoforms (L858R/T790M, resistant exon 20 insertions, R108K-resistant mutations, or EGF-stimulated wild-type EGFR) were below concentrations needed for erlotinib’s inhibitory effect. In addition, growth factor–independent transformation of Ba/F3 cells by EGFR expression was inhibited by afatinib at concentrations below corresponding erlotinib concentrations. Afatinib was also effective at inhibiting survival of erlotinib-sensitive cells (HCC827), erlotinib-resistant cells (NCI-1975), and wild-type cells (H1666). In xenograft models, EGFR phosphorylation and tumor volume decreased after 25 days of daily afatinib. In a murine L858R/T790M model, reduction in tumor volume was also shown with afatinib. In de novo L8585R/T790M tumor models, a daily dose of afatinib led to tumor reduction after 4 weeks. These studies suggested that afatinib may operate using a different mechanism to achieve EGFR inhibition, and it was hypothesized that it could provide clinical benefit where previous TKIs have failed.
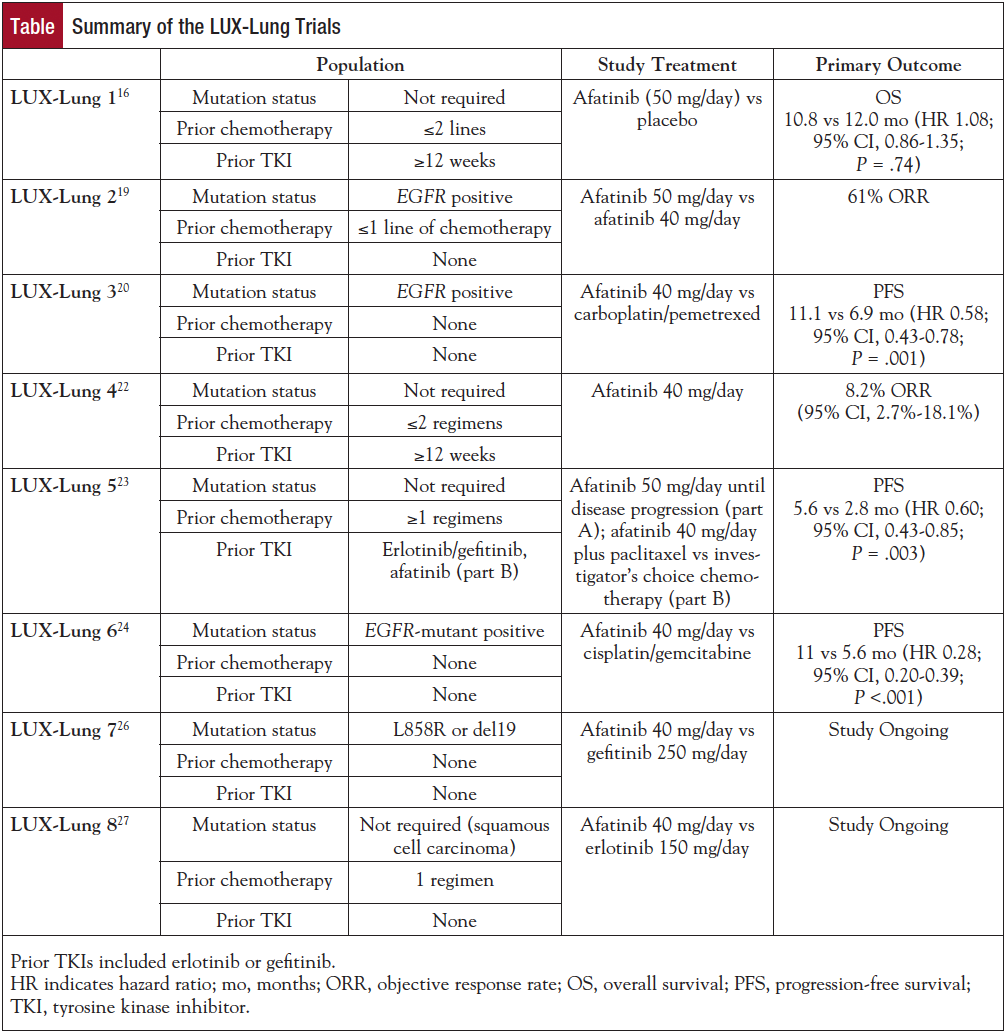
A phase 1 trial enrolled 53 patients receiving escalating doses of afatinib from 10 to 50 mg daily. The study showed an adverse event (AE) profile similar to the previous generation of TKIs, with the most common AEs being rash and diarrhea.15 The majority of patients experienced grade 1 or 2 AEs, with no grade 4 or 5 AEs occurring. Eighty-three percent of patients experienced at least 1 drug-related AE. AEs included gastrointestinal (69.8%), skin (73.6%), and general disorders, eg, mucosal inflammation and fatigue (28.3%). Diarrhea was reported by 64.2% of patients, but it was generally well controlled with antidiarrheal medications. Rash was reported in 67.9% of patients, but dry skin, hand-foot syndrome, and acne were also reported. Pharmacokinetic studies showed that afatinib concentrations were dose proportional without correlation with clearance and body size or weight. Furthermore, at steady state, trough afatinib concentrations remained above afatinib’s effective concentrations in vitro.15 The results of these early studies prompted a series of large-scale clinical trials to test the toxicity and efficacy of afatinib in NSCLC (Table).
Phase 2/3 Clinical Trials
The LUX-Lung 1 trial was a phase 2b/3 study that randomized 585 patients in a 2:1 ratio to afatinib (50 mg/day) or placebo.16 Eligible patients had to have progressed on at least 12 weeks of erlotinib or gefitinib and at least 1 prior chemotherapy regimen. Retrospective analysis of tissue samples in only 24% of patients showed that 68% were EGFR mutation positive, with the majority (79%) harboring the 2 most common mutations, L858R or del19. The primary end point was overall survival (OS), and progression-free survival (PFS), objective response rate (ORR), length of response, safety, and quality of life (QOL) were secondary end points. The primary analysis did not detect a significant difference in OS between treatment groups in the overall population (10.8 vs 12.0 months, afatinib vs placebo; P = .74) or between subgroups. When analyzed by mutation status, EGFR mutation–positive patients who received afatinib had increased PFS when compared with the EGFR mutation–positive patients who received placebo (median 3.3 vs 1.0 months; P = .009; hazard ratio [HR], 0.51). In contrast, no difference in PFS was seen in patients with less common mutations or in patients without activating EGFR mutations (median 2.8 vs 1.8 months; P = .22; HR, 0.61). Patients meeting Jackman and colleagues’ criteria for acquired resistance had a median PFS of 4.5 months on afatinib, and placebo patients had a PFS of 1.0 month.16,17 Objective responses in the afatinib arm were observed in 7% of patients by independent assessment, with responses lasting approximately 22 weeks. The QOL assessment used cough, dyspnea, and pain ratings to determine drug-related symptom improvement using the lung cancer–specific QOL questionnaire from the European Organisation for Research and Treatment of Cancer (EORTC).16 Analysis of QOL ratings showed that afatinib patients had a significantly higher incidence of improvements in NSCLC-related symptoms. In the afatinib treatment arm, 46% reported improvements in cough, 51% improvements in dyspnea, and 50% improvements in pain compared with 25%, 36%, and 32% of patients in the placebo group, respectively. The AE profile of afatinib consisted of diarrhea, rash/acne, stomatitis, itchiness, nosebleeds, and loss of appetite, with diarrhea and rash occurring in the majority of patients (17% and 14% grade 3, respectively). One hundred fifty of 390 patients (38%) on the afatinib arm required dose reduction, the majority because of diarrhea and rash. Few patients (7.6%) discontinued treatment because of drug-related AEs, indicating that dose reduction or supportive medications may be sufficient to palliate these effects.
Preclinical models, phase 1 trials, and LUX-Lung 1 established a maximum tolerated dose of 55 mg daily and a standard dose of 50 mg per day; however, because of toxicity, many patients (40% in LUX-Lung 1) needed dose reductions to 40 mg/day.14-16,18 These studies also established afatinib’s AE profile and indicated that dose reduction may be enough to lessen the severity and/or the likelihood of AEs. Study patients for LUX-Lung 2 were EGFR mutation–positive, advanced-stage adenocarcinoma patients from Taiwan and the United States who had never been treated with EGFR TKIs and had completed no more than 1 chemotherapy regimen.19 All patients received afatinib and started at either 50 mg/day (99 patients) or 40 mg/day (30 patients). The primary end point of this study was the percentage of patients with confirmed objective responses according to RECIST by independent assessment. Secondary end points included percentage of patients with disease control, time to objective response, response duration, tumor shrinkage, PFS, and OS. Median treatment duration was 12 months, with a median follow-up time of 22 months. Seventy-nine patients (61%) in the overall population had an objective response to afatinib. Notably, there was no advantage associated with treating patients with 50 mg or 40 mg of afatinib daily. Median response duration was 12.9 months, and 82% experienced disease control. Median PFS was 10.1 months, and median OS for all patients was 24.8 months. Of patients on the 50-mg dose, 28% and 22% experienced grade 3 skin events or diarrhea, respectively, but dose reduction to 40 mg significantly reduced the occurrence of these high-grade symptoms. Serious AEs occurred in less than 15% of patients in the 50-mg treatment arm, and this percentage was halved in the 40-mg treatment arm.19 Within LUX-Lung 2, the majority of patients harbored 1 of 2 EGFR mutations, L858R or a del19. Of patients with the common mutations, 66% registered an objective response compared with 39% of patients in the less common mutation subgroup. Of patients with common EGFR mutations, 88% had disease control compared with 57% of patients with other mutations. This study was one of the first to provide results about how patients with less common mutations may respond to TKI treatment.
LUX-Lung 3 was the first global study comparing afatinib with the standard systemic chemotherapy (cisplatin/pemetrexed) and demonstrated afatinib’s superiority to chemotherapy in EGFR mutation–positive patients.20 Patients (N = 345) with activating EGFR mutations were randomized to afatinib 40 mg/day or up to 6 cycles of cisplatin/pemetrexed chemotherapy every 3 weeks. Patients were treatment naive, and PFS was the primary end point. Secondary end points included ORR, disease control, safety, and AE profile. PFS for afatinib was 11.1 months compared with 6.9 months for cisplatin/pemetrexed (HR, 0.58; 95% CI, 0.43-0.78; P = .001). PFS for patients with common EGFR mutations was 13.6 months (HR, 0.47). Disease was well controlled in both treatment arms (90% with afatinib vs 81% with chemotherapy). Similar to LUX-Lung 1, cough and dyspnea symptoms were delayed with afatinib treatment (HR, 0.60; 95% CI, 0.41-0.87; P = .007 and HR, 0.68; 95% CI, 0.50-0.93; P = .015, respectively). More patients receiving afatinib reported improvements in shortness of breath (P <.001), and patients with baseline symptoms benefitted the most from afatinib treatment. Longitudinal analysis also showed that patients on afatinib had significantly better improvements in physical, role, and cognitive function.21 Overall, this was the largest study showing that afatinib could lead to significant benefits for patients with EGFR-mutant NSCLC as first-line therapy.
LUX-Lung 4 examined whether a daily 50-mg dose of afatinib could benefit patients who progressed on previous TKI treatment.22 The trial enrolled 62 patients with advanced-stage adenocarcinoma who had progressed after at least 12 weeks of erlotinib or gefitinib treatment (95.2% for at least 24.4 weeks), which served to enrich for those who developed resistance according to the Jackman criteria (82.3%).17 Seventy-two percent had an EGFR mutation in the primary tumor, and 2 patients (3.2%) developed a known T790M mutation in addition to the original activating mutation.22 Most patients discontinued treatment by the second analysis, with 64.5% discontinuing because of progression. A partial response was achieved by 8.2% of patients, 57.4% had stable disease for at least 6 weeks, and 65.6% achieved disease control. The average duration of response was 24.4 weeks, with afatinib reducing lesion size in 79% of patients.22 Median PFS was 4.4 months, and median OS was 18.4 months. There was no difference in outcomes observed when analyzing subgroups by sex, type of previous treatment, and number of chemotherapy regimens. Treatment was discontinued in 29% of patients because of AEs. Given that previous response rates were 0% to 3%, this study showed that afatinib has a modest effect in patients with acquired resistance to EGFR TKI therapy following numerous prior treatments.
LUX-Lung 5 assessed the benefit of continued EGFR inhibition after progression on afatinib.23 Part A of the study enrolled 1154 patients with advanced-stage NSCLC who had received at least 1 chemotherapy regimen and in whom erlotinib or gefitinib had failed; patients received a 50-mg daily dose of afatinib alone until disease progression. Patients who benefited from afatinib treatment could then be randomized to receive afatinib (40 mg/day) plus paclitaxel combination therapy or investigator’s choice chemotherapy in part B; 202 patients were randomized in a 2:1 ratio. Those who received afatinib plus paclitaxel had a significant improvement in median PFS over chemotherapy at 5.6 versus 2.8 months (HR, 0.60; 95% CI, 0.43-0.85; P = .003), but OS was similar in both arms. Toxicities were consistent with prior studies, and the most common AEs on the afatinib plus paclitaxel arm were diarrhea (53.8%), alopecia (32.6%), and asthenia (27.3%). This study verifies previous results that indicate afatinib’s clinical benefit in patients with EGFR-mutant NSCLC.
LUX-Lung 6 randomized 364 treatment-naive, EGFR mutation–positive patients with late-stage adenocarcinoma to afatinib 40 mg/day versus cisplatin and gemcitabine every 3 weeks.24 Confirming previous results, the majority of patients had the 2 most common EGFR mutations, with only 2.5% of the study population harboring other mutations. The researchers’ primary aim was PFS, but QOL (assessed using the EORTC QOL questionnaire), ORR, disease control, and duration of response were also evaluated. PFS was improved in the afatinib treatment arm, except in patients with uncommon EGFR mutations or those who were former smokers. Median PFS was 11 months with afatinib compared with 5.6 months with chemotherapy (HR, 0.28; 95% CI, 0.20-0.39; P <.001). Sixty-six percent of patients experienced an objective response, and duration of response was 9.7 months with afatinib compared with 23% and 4.3 months, respectively, with chemotherapy. Length of disease control was also extended in the afatinib group (11.1 vs 5.7 months). For patient-reported outcomes, the afatinib group reported more improvements in cough, dyspnea, and general pain. Time to deterioration for these symptoms was also significantly longer in afatinib-treated patients. Grade 1/2 AEs were more likely in the afatinib treatment group (62.8% vs 38.9%). This and previous studies suggest that many of the less common EGFR mutations act as activating mutations; however, a subset may be associated with a decreased response to afatinib.18,24 This study confirmed the benefit of afatinib as initial therapy for patients with EGFR mutation–positive NSCLC.
A recent pooled analysis of LUX-Lung 320 and LUX-Lung 624 was presented that included 709 patients randomized in both studies to evaluate OS in patients with exon 19 (n = 355) and exon 21 (n = 276) mutations.25 Although an improvement in PFS has been demonstrated with EGFR TKI therapy compared with chemotherapy in EGFR-mutant NSCLC for all agents investigated, an OS benefit had not previously been shown. This exploratory analysis did not show an improvement in OS with afatinib compared with chemotherapy at 25.8 versus 24.5 months (HR, 0.91; 95% CI, 0.75-1.11; P = .37) in the combined group. When separated by exon 19 versus exon 21 mutations, a survival benefit was seen specifically in those with del19 mutations (HR, 0.59; 95% CI, 0.45-0.77; P <.001). The combined analysis provides hypothesis-generating results but is limited by multiple testing with data from 2 separate trials with differing populations. A number of patients enrolled did not receive EGFR TKI therapy for EGFR-mutant NSCLC, which may influence the results, and these data were not available for the analysis. This study does suggest that EGFR-directed therapies may have differing outcomes based on the specific EGFR mutation.
Afatinib has yet to show how it differs from the previous generation of EGFR treatments. Both inhibit EGFR function and show benefits for PFS and ORR over chemotherapy, but resistance and variability of response within mutation type remain an issue. An ongoing trial, LUX-Lung 7, will help to assess afatinib compared with gefitinib to determine whether afatinib may improve PFS and OS over first-generation EGFR TKIs.26 LUX-Lung 8 is assessing afatinib versus erlotinib in patients with squamous cell carcinoma, although this population has demonstrated limited benefit with EGFR TKI therapy.27
Acquired Resistance
Through its differential mechanism of irreversible binding, afatinib was thought to provide a potential benefit over reversible EGFR inhibitors to slow or even stop the development of T790M gatekeeper mutations, although this has not been demonstrated in a clinical setting. Like afatinib, dacomitinib is another irreversible pan-HER inhibitor that has shown promise in preclinical models. Unfortunately, this second-generation TKI has not established clinical efficacy against T790M.13,28 Benefits for those with acquired resistance are still minimal, and alternative therapeutic strategies are being explored. Cell line studies have shown that higher doses of afatinib are needed to inhibit EGFR in resistant cells, so there is a possibility that an effective dose against T790M exceeds the maximum tolerated dose of 55 mg. To answer this need, combination treatment with afatinib and the EGFR monoclonal antibody cetuximab has been evaluated and led to clinical benefit.29 Studies in preclinical models of EGFR TKI resistance due to T790M demonstrated efficacy with the combination.30 A phase IB trial showed a promising response rate of 32% with an 8-month median duration of response in patients with EGFR-mutant lung cancer with EGFR TKI resistance.29 Genomic analysis of patient tumor samples found alteration in NF2 and TSC1, which modulate mTOR signaling as a potential pathway of resistance in this population.31 Third-generation, specific T790M inhibitors that target mutant EGFR and T790M, but largely spare wild-type receptors, also show early clinical efficacy in those who develop resistance due to the T790M mutation.32,33
Conclusion
Targeted treatments have proved to be a major advancement in the fight against cancer. Patients with EGFR mutant–positive advanced-stage lung cancer who are treated with EGFR TKIs have a survival rate double what it was previously with chemotherapy alone. Adverse effects of afatinib are similar to those seen with the previous generation of inhibitors, and its approval supports the use of EGFR TKIs in this setting. While more is being done to further improve efficacy and combat resistance to EGFR TKIs, afatinib offers additional options for first-line therapy and is a step forward in the treatment of patients with EGFR-mutant NSCLC.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139.
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957.
- Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760-774.
- Veale D, Kerr N, Gibson GJ, et al. Characterization of epidermal growth factor receptor in primary human non-small cell lung cancer. Cancer Res. 1989;49:1313-1317.
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380.
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812-3821.
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31:1070-1080.
- Yu HA, Pao W. Targeted therapies: afatinib – new therapy option for EGFR-mutant lung cancer. Nat Rev Clin Oncol. 2013;10:551-552.
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73.
- Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695-2706.
- Mok T, Lee K, Tang M, et al. Dacomitinib for the treatment of advanced or metastatic non-small-cell lung cancer. Future Oncol. 2014;10:813-822.
- Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3337-3344.
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702-4711.
- Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965-3972.
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528-538.
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357-360.
- Marshall J, Hwang J, Eskens FA, et al. A phase I, open-label, dose escalation study of afatinib, in a 3-week-on/1-week-off schedule in patients with advanced solid tumors. Invest New Drugs. 2013;31:399-408.
- Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012;13:539-548.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-3334.
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342-3350.
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3341.
- Schuler MH, Yang CH, Park K, et al. Continuation of afatinib beyond progression: results of a randomized, open-label, phase III trial of afatinib plus paclitaxel (P) versus investigator’s choice chemotherapy (CT) in patients (pts) with metastatic non-small cell lung cancer (NSCLC) progressed on erlotinib/gefitinib (E/G) and
afatinib – LUX-Lung 5 (LL5). J Clin Oncol. 2014;32(suppl). Abstract 8019.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-222.
- Yang JCH, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and Lux-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141-151.
- LUX-Lung 7: a phase IIb trial of afatinib (BIBW2992) versus gefitinib for the treatment of 1st line EGFR mutation positive adenocarcinoma of the lung. Clinical Trials.gov website. www.clinicaltrials.gov/ct2/show/NCT01466660. Published November 4, 2011. Updated July 9, 2014. Accessed July 18, 2014.
- LUX-Lung 8: a phase III trial of afatinib (BIBW 2992) versus erlotinib for the treatment of squamous cell lung cancer after at least one prior platinum based chemotherapy. ClinicalTrials.gov website. www.clinicaltrials.gov/ct2/show/NCT01523587. Published January 30, 2012. Updated July 9, 2014. Accessed July 18, 2014.
- Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120:1145-1154.
- Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036-1045.
- Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000-3010.
- Pirazzoli V, Nebhan C, Song X, et al. Acquired resistance of EGFR-mutant lung adenocarcinomas to afatinib plus cetuximab is associated with activation of mTORC1. Cell Rep. 2014;7:999-1008.
- Sequist LV, Soria JC, Gadgeel SM. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M). J Clin Oncol. 2014;32(suppl). Abstract 8010.
- Janne PA, Ramalingam SS, Yang JCH, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor–resistant non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32(suppl). Abstract 8009.
Ms Tsui is a sophomore at Duke University majoring in biology and minoring in chemistry. She was selected as a CURE student for the City of Hope Eugene and Ruth Roberts 12-week Summer Student Academy where she performed research on EGFR mutations in lung cancer and their association with genetic susceptibility for cancer.
Dr Reckamp is Associate Professor in the Department of Medical Oncology and Therapeutics Research at City of Hope. She received her medical degree from the University of Chicago and her master’s degree in Clinical Investigation from UCLA. She completed residency training in Internal Medicine at Barnes-Jewish Hospital and a Hematology/Oncology fellowship at the David Geffen School of Medicine at UCLA.
Her activities at City of Hope include Medical Director of the Thoracic Oncology program and Chair of the COHCCC scientific review committee. She is also a member of the NCCN Lung Cancer Guidelines Committee and a principal investigator for many phase 1 and 2 studies funded by the National Cancer Institute.

