Multiple myeloma (MM) is a malignancy of plasma cells that accumulate in the bone marrow and/or extramedullary sites, leading to complications such as renal failure, bone destruction, and hypercalcemia.1 Recent treatment advancements in MM have led to improvements in patient outcomes.2 In general, 3 drug classes are used in the management of MM, including proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies (specifically, those targeting CD38); in addition, autologous stem-cell transplantation (ASCT) is used in eligible patients.2,3 Despite these advancements in treatment, the journey of patients with MM is characterized by multiple remissions and relapses, and the disease remains incurable.
Numerous considerations must be acknowledged when seeking to optimize the therapeutic choices for patients with relapsed/refractory MM (RRMM), including patient-specific factors and the context of the clinical relapse, such as the class of agents previously administered.1-3 The subgroup of patients who are refractory to the 3 standard drug classes (triple-refractory patients) face a particularly poor prognosis, with overall survival of ≤7 to 9 months.3 Although a standard of care has yet to emerge, current treatment approaches include conventional chemotherapy, salvage ASCT, and selinexor, underscoring the urgent need for new drugs with novel mechanisms of action.3,4 In this context, B-cell maturation antigen (BCMA) has emerged as a novel target for therapies with immunologically mediated action in MM, considering BCMA is a specific myeloma-associated antigen.5-7
Novel BCMA-Directed Treatment Strategies
BCMA belongs to the tumor necrosis factor receptor superfamily 17 and is ubiquitously expressed on malignant plasma cells and some subsets of mature B-cells. BCMA regulates B-cell proliferation, survival, maturation, and differentiation, thus making it an ideal target antigen for therapeutic intervention.5-8
Several BCMA-targeted strategies are being investigated in RRMM, including antibody–drug conjugates (ADCs), bispecific T-cell engagers (BiTEs), and adoptive cell therapy such as chimeric antigen receptor (CAR) T-cell therapies (Figure 1).5-8 BiTEs are molecules with affinities for 2 different epitopes, BCMA on MM cells and CD3 on host T-cells, that target T-cell effector function to induce cytotoxicity of BCMA-expressing MM cells (Figure 2).5,7,9
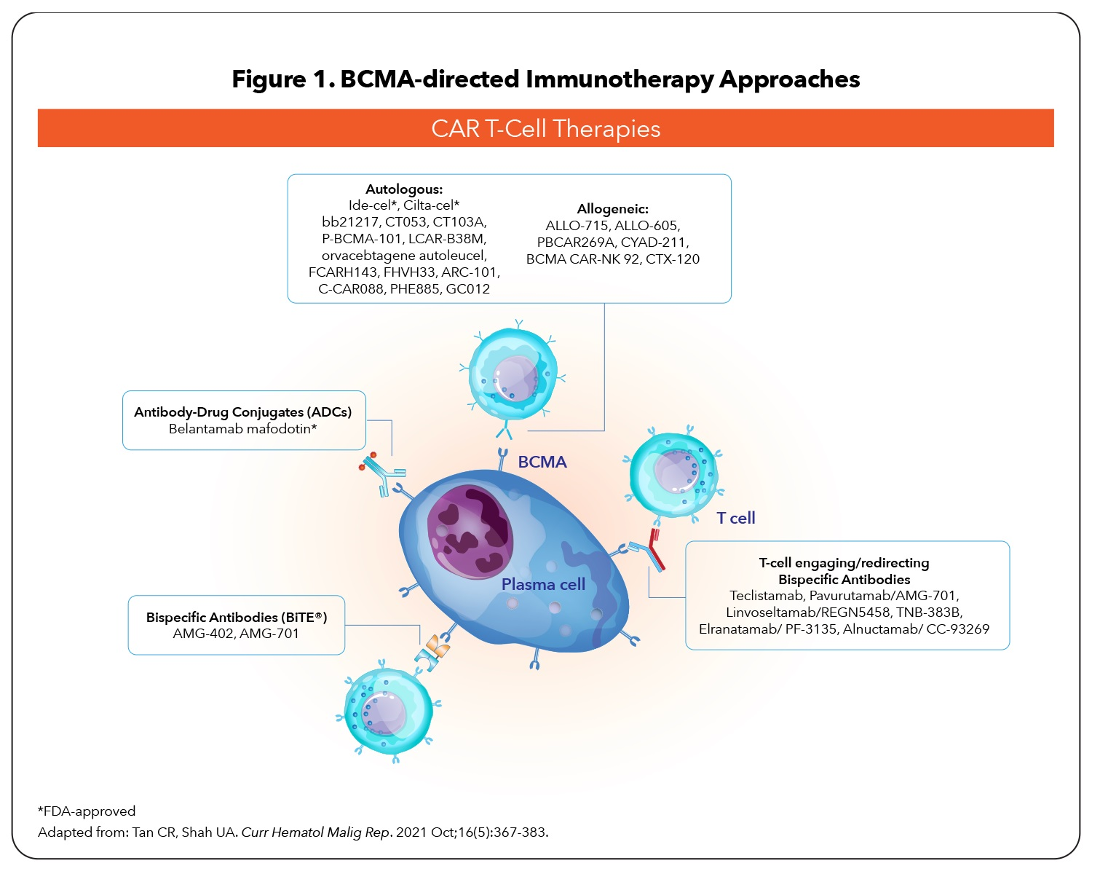
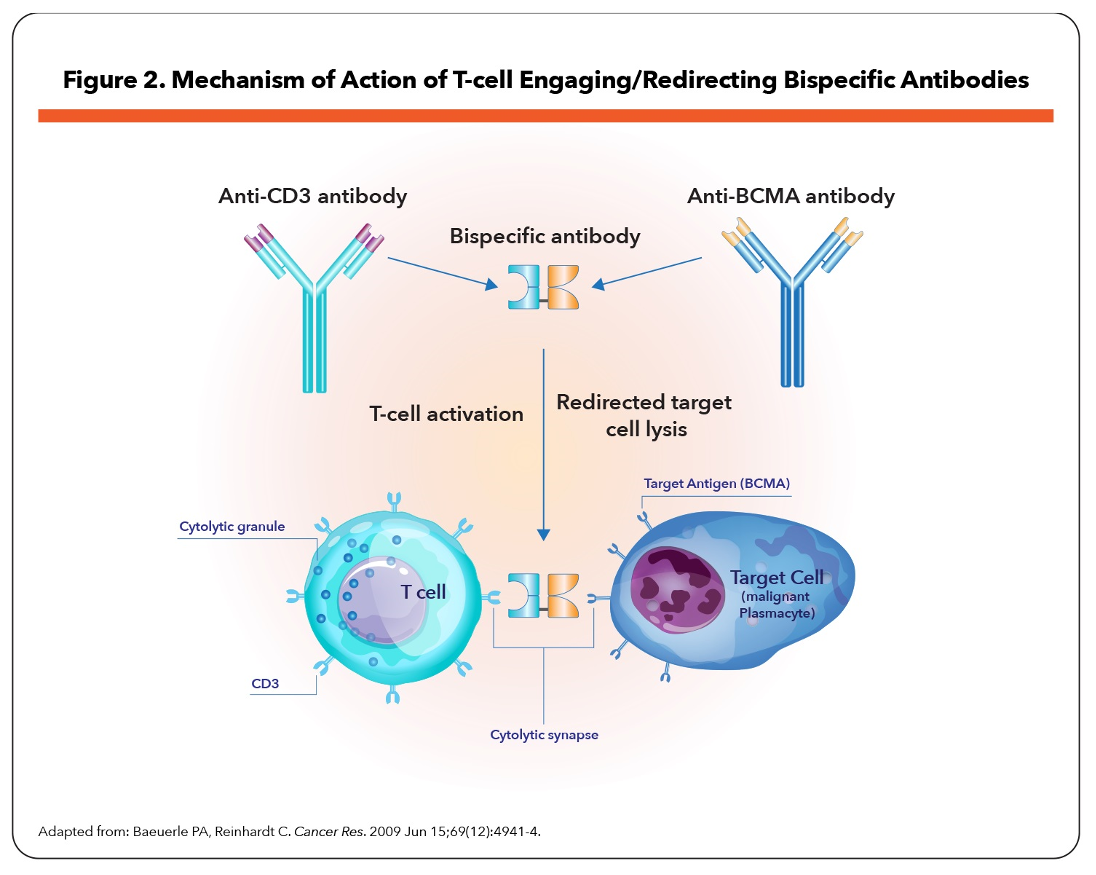
Clinical evidence indicates unprecedented antimyeloma activity with BCMA modalities in highly pretreated RRMM patients,5,7,8,10-12 leading to the recent approvals of 3 agents: belantamab mafodotin (ADC), idecabtagene vicleucel (CAR T-cell therapy), and ciltacabtagene autoleucel (CAR T-cell therapy).13-15
Key differences in the BCMA-targeted modalities are associated with profound clinical implications; these differences involve production time, administration routes, toxicities, and immune-expansion capabilities.6,7,10 Although CAR T-cell therapy is not human-leukocytes-antigen-restricted, disadvantages include longer production time, manufacturing expense, bridging with chemotherapy, and need for prolonged hospitalization in specialized centers.6,7,10 BiTEs have advantages: available as “off-the-shelf” reagents, more favorable safety profile, and subcutaneous (SC) administration; however, the main limitation is their short duration of effect, leading to the need for continuous administration.7,10
Toxicities seen with BiTEs and CAR T-cell therapies mainly consist of cytokine release syndrome (CRS), immune effector-cell–associated neurotoxicity syndrome (ICANS), cytopenia, and infections.7,10 The majority of these adverse reactions can be managed with current mitigation protocols but require a significant degree of expertise and familiarity with these classes of therapeutics. Principles of management of CRS include premedication with corticosteroids, including methylprednisolone or dexamethasone, and use of IL-6 receptor antagonists such as tocilizumab; supportive care includes oxygen, fluid boluses, anti-infective agents, and vasopressors.16
Investigational BCMA-Targeting BiTEs in Clinical Testing
Currently, several BiTEs target BCMA on MM cells and CD3 receptors on T-cells; these agents are in various stages of clinical testing; safety and efficacy results from early-phase trials of these agents are presented in the Table.
Table. Key Clinical Data from Studies Investigating BCMA-Targeting BiTE Antibodies
| Agent | How Administered | Study Population; | Efficacy | Safety |
|---|---|---|---|---|
| Teclistamab18 |
|
|
|
|
| Pavurutamab/ AMG-70119 |
|
|
|
|
| Linvoseltamab/ REGN545820 |
|
|
|
|
| TNB-383B21 |
|
|
|
|
| Elranatamab/PF-313522,23 |
|
|
|
|
|
|
|
|
|
| Alnuctamab/CC-9326924 |
|
|
|
|
| BCMA indicates B-cell maturation antigen; CR, complete response; CRS, cytokine release syndrome; esc, escalation; exp, expansion; ICANS, immune effector-cell–associated neurotoxicity syndrome; IMiD, immunomodulatory drug; IV, intravenous; MRD-neg, negative for minimal residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PL, prior lines (of therapy); Q2W, every 2 weeks; Q3W, every 3 weeks; Q4W, every 4 weeks; RP2D, recommended phase 2 dose; SC, subcutaneous; VGPR, very good partial response. | ||||
Teclistamab
Teclistamab is being investigated in the ongoing MajesTEC-1 trial.17,18 In the phase 1/2 segment,18 165 patients with RRMM (median of 5 prior lines of therapy [LOT]; 77.8% triple-refractory) received a weekly SC injection of teclistamab at the recommended phase 2 dose (RP2D) of 1.5 mg/kg (following step-up doses of 0.06 mg/kg and 0.3 mg/kg). At a median follow-up of 14.1 months, the overall response rate (ORR) was 63.0%, with 39.4% achieving complete response or better (≥CR) and 26.7% with no minimal residual disease. The median progression-free survival was 11.3 months. CRS occurred in 72.1% of patients (grade 3/4, 0.6%), infections in 76.4% (grade 3/4, 44.8%), and neurotoxic events in 14.5%, including ICANS, in 3.0% (all grade 1/2).
Pavurutamab (AMG-701)
Pavurutamab is in phase 1 clinical testing in patients with RRMM who had received ≥3 prior LOTs (median of 6 prior regimens; 93% had received prior anti-CD38 antibody, 62% were triple-refractory, 83% had prior ASCT).19 Patients (N = 85) received intravenous (IV) pavurutamab weekly in 4-week cycles; a step dose of 0.8 mg was administered before target doses ≥1.2 mg. CRS occurred in 65% of patients and most were grade 1/2; grade 3 CRS was reported in 8 patients, and these events were reversible with corticosteroids and tocilizumab. ORR was 26% in the efficiency-evaluable cohort (n = 82), including a very good partial remission or better (≥VGPR) of 17%; in the most recent evaluable cohort (n = 6), ORR was 83%, with ≥VGPR of 50%. Of note, a phase 1b, open-label study evaluating the safety and pharmacokinetics of pavurutamab administered through the SC route for the treatment of patients with RRMM, called ProxiMMity-1 (NCT04998747), has been withdrawn by the manufacturer/sponsor for business (rather than safety) reasons.
Linvoseltamab (REGN5458)
Linvoseltamab is being investigated in an ongoing, 2-part, phase 1/2 trial (NCT03761108) in heavily pretreated patients with RRMM.20 Of the 73 patients who received linvoseltamab in 3-mg to 800-mg dose levels, 38% were penta-refractory and 89% were triple-refractory. At higher dose levels (200-800 mg), an ORR of 75% was achieved including a ≥VGPR rate of 58%. CRS occurred in 38% of patients; there were no grade ≥3 CRS cases or discontinuations due to CRS. ICANS was reported in 3 patients (4%). The phase 2 portion of the study is currently recruiting.
TNB-383B
TNB-383B is a BiTE that engages BCMA and CD3 receptors but has a low-affinity CD3 domain designed to evade systemic T-cell activation to minimize CRS. The ongoing phase 1 dose-escalation/expansion study is investigating TNB-383B (0.025-120 mg IV every 3 weeks) in patients with RRMM (median of 5 prior LOT; 61% triple-refractory).21 The dose-escalation phase (n = 73) established the RP2D to be 60 mg every 3 weeks. In the dose-escalation cohort receiving ≥40 mg every 3 weeks (n = 24), an ORR of 79% was achieved (median follow-up, 8.0 months), with ≥VGPR achieved in 63%. In the dose-escalation plus dose-expansion cohort (n = 45; total N of 118 patients in this study), ORR was 60% (median follow-up, 4.3 months), with ≥VGPR achieved in 43% of patients, while complete response (CR) in patients receiving higher doses (>40 mg every 3 weeks) reached 20%. Treatment-related CRS was reported in 54% of patients (grade 3, 3%); infections occurred in 28%.
Elranatamab (PF-3135)
Elranatamab was evaluated in the first-in-human phase 1 MagnetisMM-1 study22 (NCT03269136) that demonstrated an ORR of 64% (median follow-up, 8.1 months) with elranatamab (80-1000 mg/kg every week or every 2 weeks) in patients with RRMM (N = 55; median prior LOT, 6; triple-class refractory, 91%; prior ASCT, 56%; prior BCMA-targeted therapy, 22%). Using a single priming dose (600 μg/kg) and the RP2D (1000 μg/kg), CRS occurred in 67% of patients, with no grade 3/4 CRS.
The open-label, nonrandomized, phase 2 MagnetisMM-323 (NCT04649359) study enrolled patients (n = 94) who are triple-refractory (median of 5 prior LOTs, 96% were triple-refractory, and 39% were penta-drug refractory). In addition, patients were either naïve to BCMA-directed therapies (cohort A) or had previous exposure to BCMA-directed ADCs or CAR T-cell therapy. Patients received SC elranatamab 1000 μg/kg weekly on a 28-day cycle; a 2-step-up fixed-dose priming regimen (12 mg or 32 mg) was used instead of the single priming dose of 600 μg/kg to mitigate CRS. In all treated patients, an ORR of 61% was achieved. Among patients who received the 2-step-up priming regimen (n = 90), CRS occurred in 58.9% (all grade 1/2) and ICANS in 2.2%.
Alnuctamab (CC-93269)
Alnuctamab is being evaluated in an ongoing phase 1 trial24 (NCT03486067) at a dose range of 0.15 mg to 10 mg (weekly for cycles 1 to 3, every other week for cycles 4 to 6, and every 4 weeks for cycle 7 and beyond) in heavily pretreated patients (n = 30; median prior LOT, 5) who were all refractory to the last line of therapy. An ORR of 43% was achieved in the total cohort (CR/≥CR, 17%) and 89% (CR/≥CR, 44%) in the 10 mg cohort (n = 9). CRS occurred in 77% of patients, mostly grade 1/2; 1 death was reported in a patient with CRS and infection.
Conclusions
The treatment paradigm of RRMM continues to evolve at a rapid pace, with targeted immunotherapy playing a major role. The advent of BCMA-targeting therapies represents a new era in immunotherapy for RRMM. In particular, BCMA-targeted BiTEs are “off-the-shelf” therapeutic products, ie, without need for ex vivo manipulation of MM cells and complex manufacturing and distribution processes, that have demonstrated promising efficacy and acceptable safety, supporting ongoing evaluation and further clinical development. Current research efforts are investigating BCMA-targeted BiTEs in earlier LOTs, as well as in combination as a doublet of these agents targeting 2 different antigens—to prevent antigen escape—or with other active antimyeloma drugs (such as a CD38-targeting antibody or an immunomodulatory drug).
References
- Callander NS, Baljevic M, Adekola K, et al. NCCN Guidelines® insights: multiple myeloma, version 3.2022. J Natl Compr Canc Netw. 2022;20(1):8-19.
- Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020;10(9):94.
- Hernández-Rivas J-A, Ríos-Tamayo R, Encinas C, et al. The changing landscape of relapsed and/or refractory multiple myeloma (MM): fundamentals and controversies. Biomark Res. 2022;10(1):1.
- Mikhael J. Treatment options for triple-class refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020 Jan;20(1):1-7.
- Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020;13(1):125.
- Nobari ST, Nojadeh JN, Talebi M. B-cell maturation antigen targeting strategies in multiple myeloma treatment, advantages and disadvantages. J Transl Med. 2022;20(1):82.
- Kleber M, Ntanasis-Stathopoulos I, Terpos E. BCMA in multiple myeloma—a promising key to therapy. J Clin Med. 2021;10(18):4088.
- McClanahan A, Spychalla M. New frontiers: the role of CAR T-cell therapy in multiple myeloma. J Adv Pract Oncol. 2022;13(3):328-332.
- Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69(12):4941-4944.
- Sanchez L, Dardac A, Madduri D, et al. B-cell maturation antigen (BCMA) in multiple myeloma: the new frontier of targeted therapies. Ther Adv Hematol. 2021;12:2040620721989585.
- Swan D, Routlegde D, Harrison S. The evolving status of immunotherapies in multiple myeloma: the future role of bispecific antibodies. Br J Haematol. 2022;196(3):488-506.
- Tan CR, Shah UA. Targeting BCMA in multiple myeloma. Curr Hematol Malig Rep. 2021;Oct;16(5):367-383.
- FDA. FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma. Accessed August 9, 2022.
- FDA. FDA approves first cell-based gene therapy for adult patients with multiple myeloma. www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-multiple-myeloma. Accessed August 9, 2022.
- FDA. FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma. Accessed August 9, 2022.
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330.
- Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398(10301):665-674.
- Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495-505.
- Harrison SJ, Minnema MC, Lee HC, et al. A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE® (bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM). Blood. 2020;136:28-29.
- Zonder JA, Richter J, Bumma N, et al. Early, deep, and durable responses, and low rates of CRS with REGN5458, a BCMAXCD3 bispecific antibody, in a phase 1/2 first-in-human study in patients with relapsed/refractory multiple myeloma. Presented at: 2022 EHA Congress; Abstract S189.
- Kumar S, D’Souza A, Shah N, et al. A phase 1 first-in-human study of Tnb-383B, a BCMA x CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. Blood. 2021;138(suppl 1):900.
- Jakubowiak AJ, Bahlis NJ, Raje NS, et al. Elranatamab, a BCMA-targeted T-cell redirecting immunotherapy, for patients with relapsed or refractory multiple myeloma: updated results from MagnetisMM-1. J Clin Oncol. 2022;40(16 suppl):8014-8014.
- Lesokhin AM, Arnulf B, Niesvizky R, et al. Initial safety results for MagnetisMM-3: a phase 2 trial of elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM). J Clin Oncol. 2022;40(suppl 16):8006-8006.
- Costa JL, Wong SW, Bermudez A, et al. Interim results from the first phase I clinical study of the B-cell maturation antigen (BCMA) 2 +1 T-cell engager (TCE) CC-93269 in patients with relapsed/refractory multiple myeloma. Presented at: 2020 EHA Congress; Abstract S205.

